Molecular Weight Determination of Unfractionated Heparin
Wyatt Technology Corporation
Heparin is a polydisperse, heterogeneous polysaccharide derived from animal tissue. Heparin has been used as an anticoagulant for over 60 years and one of the fundamental parameters for characterizing unfractionated heparin (UFH) is its molecular weight (MW) and MW distribution. The molecular weight of heparin ranges from 3 to 50 kDa, but, typically falls within the 10 to 20 kDa range for unfractionated heparins used in medical applications. In light of heparin contamination issues in 2008, USP has proposed to include heparin molecular weight determination for the stage 3 heparin monograph revisions.
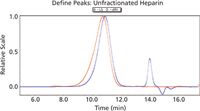
Figure 1: Examples of light scattering and RI traces for heparin.
Many high-performance size-exclusion chromatography (SEC) systems have been developed and represent the most commonly used method in the MW determination of UFH. At Scientific Protein Laboratories (SPL) we have developed an SEC system coupled with multi-angle light scattering (MALS) for the determination of absolute MW and MW distribution of UFH and heparin derivatives. MALS eliminates the need for well characterized molecular weight standards derived from heparin, and our procedure represents an improvement over the column calibration techniques used in other relative MW determination methods.
For this application note, this method uses an Agilent LC-1200 series HPLC, 0.1 M ammonium acetate mobile phase, Shodex SB-804HQ analytical column (8.0 × 300 mm) with Shodex SB-G guard column, miniDAWN TREOS light scattering detector, and Optilab rEX refractive index detector. In addition to UFH, the method is capable of analyzing dermatan sulfate and over-sulfated chondroitin sulfate (OSCS) and can distinguish among the three (Figures 1 and 2). The specific refractive index increment (dn/dc) and second virial coefficient (A2) measurements can also be determined using the Wyatt Optilab rEX and Wyatt miniDAWN TREOS detectors, respectively.

Figure 2: Linear differential molar mass graph overlay of unfractionated heparin, dermatan sulfate, and over-sulfated chondroitin sulfate (OSCS). The structural heterogeneity of glycosaminoglycans is demonstrated by the overlapping molecular weight distributions of the three samples.
Wyatt Technology Corporation
6300 Hollister Avenue, Santa Barbara, CA 93117
Tel. +1 (805) 681-9009, fax +1 (805) 681-0123
E-mail: info@wyatt.com Website: www.www.wyatt.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















