Modern Sample Preparation Methods for Food and Environmental Laboratories
Traditional sample preparation method development can often be laborious and costly. Understanding the underlying concepts of the technique can help food and environmental laboratories develop methods in notoriously complex matrices, faster, more efficiently, and provide better chromatography. With the presence of many unique matrices and analytes, methods such as QuEChERS, supported liquid extraction (SLE), or solid-phase extraction (SPE) provide the necessary adaptability for many types of extractions. With customizable methods to work with unique matrices and with the addition of automation, extractions can be improved to save time and provide consistent recoveries.
F16-ISO100/stock.adobe.com

Traditional sample preparation method development can often be laborious and costly. Understanding the underlying concepts of the technique can help food and environmental laboratories develop methods in notoriously complex matrices, faster, more efficiently, and provide better chromatography. With the presence of many unique matrices and analytes, methods such as QuEChERS, supported liquid extraction (SLE), or solid-phase extraction (SPE) provide the necessary adaptability for many types of extractions. With customizable methods to work with unique matrices and with the addition of automation, extractions can be improved to save time and provide consistent recoveries.
Method development can be the most difficult part of a laboratory’s function because of the added time, cost, and delay to productive workflows. This is made even more problematic because most food and environmental laboratories do not work with a single analyte or matrix. This means that sample preparation methods must be adaptable, flexible, and help to produce good chromatography-the ultimate reason to perform sample preparation. With lower limits of detection and a diverse list of pesticides, per- and polyfluoroalkyl substances (PFASs), and other residues to analyze, there is even more pressure for laboratories to produce sample preparation methods in a short amount of time. With basic sample preparation knowledge and help of automation, laboratories can take traditional methods and build upon them for their unique analytes to produce semiâcustomized solutions.
Working with proteins, fats, sugars, vegetables, soil, different types of water, oils, and flowers begs the question: Can there be a one size fits all sample preparation technique?
Unfortunately, there is not one method that will work for everything, but understanding the contributing chemical factors in existing methods propels the analyst to be better equipped to select and refine an effective method. Easily implemented techniques with minimal method development are preferred, especially when they allow for customization to achieve even better results.
Techniques such as QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe), supported liquid extraction (SLE) ,and solid-phase extraction (SPE) are all good options when working with diverse food and environmental matrices. The process is made even easier by incorporating benchtop processing or automation to reduce the time it takes to process large numbers of samples. With the proper tools, method development should be easy, painless, and lead to accurate liquid chromatography (LC) or gas chromatography (GC) results.
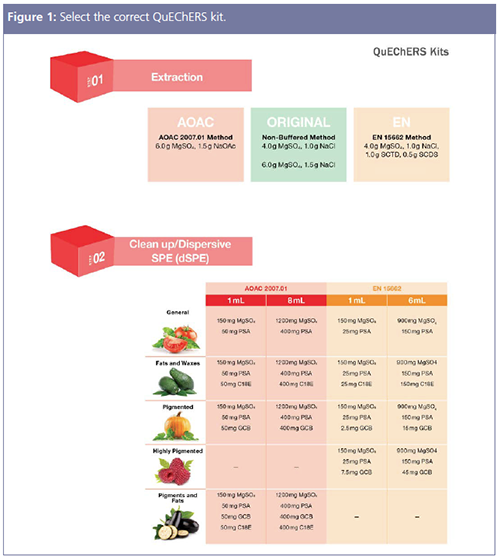
As mentioned previously, there is a lot of variation with food and environmental samples so a helpful place to start is with a technique that requires minimal adjustments but can adapt to a tailored solution for multiresidue pesticides, PFASs, pyrethroids, pharmaceuticals and personal care products (PPCPs), and mycotoxins from a plethora of diverse matrices. QuEChERS is one of the most common types of extraction techniques in food laboratories, combining extraction and clean-up steps in a convenient kit. To select the correct apparatus, it must be determined what type of extraction method is required, such as EN or AOAC methods, and then which dispersive SPE (dSPE) method is the most appropriate for the sample matrix. For example, if working with a pigmented sample, the use of AOAC 2007.01 Method would be an excellent starting point. With AOAC 2007.01, the extraction step uses a combination of magnesium sulphate (MgSO4) salts to induce phase separation between water content in the sample and an acetonitrile layer, while sodium acetate (NaOAc) acts as a buffer to control the pH. Figure 1 displays that the dSPE method for the AOAC 2007.01 Method includes a sorbent combination of MgSO4, primary/secondary amine (PSA), and graphitized carbon black (GCB). In the dSPE portion of the protocol (for pigmented samples), the MgSO4 removes excess water from the sample, the PSA removes organic acids, fatty acids, sugars, and anthocyanin pigments, while the GCB removes pigments from the sample. As most QuEChERS kits are based on sample size, selection of the extraction salts and dSPE sorbent is still easy and quick. While traditional QuEChERS methods were for multiresidue clean-up, this approach can now be modified to allow for the expansion from the typical analysis, especially with recent interest in solid and semi-solid environmental samples, such as soil and sediments, and with the analysis of newer and emerging analytes such as PFAS. With recent food safety outbreaks, quick methods have been developed to test and help mitigate contaminated food, such as the extraction of fipronil, amitraz, and metabolites from egg albumin using QuEChERS and liquid chromatography with tandem mass spectrometry (LC–MS/MS) (1). With the ability to complete fast method development using QuEChERS, rapid implementation and swift results mitigate food safety outbreaks.
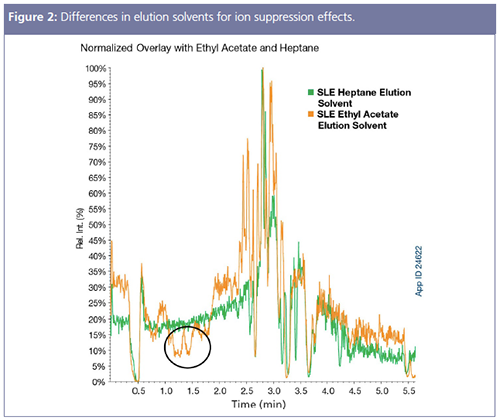
Supported liquid extraction (SLE) is one of the most selective sample preparation techniques and one of the easiest to develop a method on. The basics of SLE are the same as liquid–liquid extraction (LLE), but SLE adds improvements by eliminating emulsions, facilitating high-throughput workflows, allowing for automation, while ultimately providing more reliable and consistent results. Since SLE is a two-step protocol, samples are introduced in an aqueous or polar organic solution and then a nonpolar organic, immiscible solvent is selected for elution. Multiple solvents and different concentrations can be screened for extraction efficiency in less than 15 min and then analyzed for matrix effects, ion suppression, and overall recovery and reproducibility.
Figure 2 displays differences in the extraction solvent selection for samples in a LC–MS/MS method, showing that ethyl acetate has areas of ion suppression zones that could negatively affect the analysis if analytes are eluting in this region. Not only does selecting the wrong elution solvent cause signal suppression, in this case, using ethyl acetate lengthens dry down times (2). As a result of the ease of extraction solvent screening, many laboratories working with aqueous matrices will start with SLE before trying SPE because it saves unnecessary time spent on method development and results in a faster two-step method that can be easily implemented in the laboratory. In certain conditions, SLE results in cleaner extracts with reduced ion suppression and matrix effects when compared to SPE. Sample volumes determine the proper SLE tube size, leading to large tubes being the common SLE format for common food and environmental sample matrices, such as coffee, tea, and water. Even nonsoluble matrices, such as oils, can be compatible with SLE if mixed with a strong organic, dried down, and reconstituted with deionized (DI) water (3).
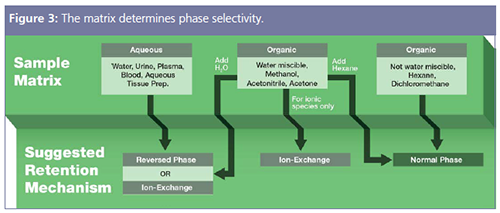
Solid-phase extraction (SPE) is often considered difficult in terms of method development, but it is also the most customizable option because of the ability of the SPE sorbent to selectively bind with the analytes of choice, making the time spent on method development acceptable for highly selective results and recoveries. Understanding how to select the proper sorbent and the recommended starting methods provides the foundation to tailor methods for individual needs and analytes. In addition, many SPE vendors offer advanced method development tools on their websites to help with sorbent selections. To start the sorbent selection, the characteristics of the sample matrix need to be determined. Figure 3 displays how the sample matrix funnels into a suggested retention mechanism for SPE, then the phase selection will follow based on analytes of choice.
Two common types of SPE sorbents that exist are silica and polymeric SPE. Silicaâbased SPE sorbents are the backbone of traditional SPE. They offer a wide range of extraction possibilities that can go from general, such as C18 or unbonded silica, to analyte-specific extractions, such as activated magnesium silicate for the retention of polar and halogenated compounds, extractable petroleum hydrocarbons (EPH) for fractionation of aliphatic and aromatic hydrocarbons from environmental samples, or GCB for removing pigments or improving recovery when working with pesticides from water, fruits, and vegetables. However, polymeric SPE is a cleaner and more retentive option that separates the stationary phases by broader categories of analytes, such as bases, neutrals, and acids, alongside three different mechanisms of retention. After selecting the proper phase, vendors offer starting methods to get the extraction underway. If running a recommended vendor method, keep in mind that pretreatment, washes, and the flow rate are small but integral steps of a SPE method that have a large impact on the recoveries and can often be overlooked.
Laboratories often follow the recommended starting method correctly and still don’t understand why they keep achieving poor recoveries-a source of most of the frustration with SPE. If the first couple of extractions don’t go as planned, evaluate the process step-by-step to understand where things could have gone wrong. Start from the beginning with questions such as: Is the sorbent selection correct? Did I use the correct flow rates on critical steps? Am I using the correct wash or elution solvents? The best place to start is to first determine if the chosen sorbent is correct, and if it is, analyze the fractions to understand where the analyte is present:
- If the analyte is coming out after loading the sample onto the cartridge this is a result of incomplete bonding to the SPE stationary phase, which could mean that the phase selection is not correct, that the sample solvent is too strong for the conditioning solvents, or that the loading solution pH needs to be adjusted.
- If the analytes are present in the wash solvents, this usually means that the wash solvent is too strong, or being ran at the incorrect pH, or that the sorbent mass is too low.
- If the analyte cannot be found, this may be because the analytes are still bonded to the SPE sorbent and a stronger elution or higher percent of organic ratio may be necessary to achieve accurate results.
The answer to the above questions should help to determine what may have gone wrong, so the next step is to adjust the necessary wash or elution solvents and run the extraction again. Figure 4 shows a detailed account of how to determine the proper wash or elution solvent that works to remove the interferences yet leads to good recoveries, which sometimes may be a necessary step before continuing the method development process. For larger classes of analytes, such as pesticides, getting a method that elutes all analytes from the sorbent may be more difficult, and elution or wash solvents may need to be selected that slightly compromise the cleanliness, but result in good recovery. If questions remain about the process, or
it gets to a point where it feels overwhelming, many SPE resources are out there such as webinars, whitepapers, videos, method development technical notes, and specific analyte technical notes to help ease the method development headaches.

Additional ways to save time and further improve results on food or environmental samples is to implement some type of automation. After a method is developed, automation can make it easier to get faster and more accurate results, especially with large sample sets. Liquid handling systems are great, but are a large investment that take a lot of due diligence and time to evaluate before purchasing. Positive pressure manifolds (PPM) are a suitable alternative to a liquid handler because they are less expensive but can help regulate the process and provide more accurate results from tube to tube. PPMs push the gas source from above, so if a tube clears before the others, it does not affect the flow rate of the other tubes. Meanwhile, vacuum manifolds cause the other wells to move faster if a well clears early, which could negatively affect results downstream. Some PPMs are now compatible with different types of tubes using a single manifold, further improving the adaptability of a single unit. These small improvements in the handling of a sample often have a large impact on workflows, saving time and providing more consistent results.
While sample preparation techniques may be slowly evolving, methods are not, and methods need to be developed based on diverse sample matrices and different analytes with chemical compatibilities. Techniques such as QuEChERS are traditionally used for multiresidue pesticide analysis from solid food or environmental samples because the dSPE clean-up step targets the matrix rather than needing a retentive clean-up that targets hundreds of analytes with a diverse range of chemical and physical properties. SLE is a great option for aqueous samples. It is easy to screen elution solvents, and with correct eluent the recoveries are high with low matrix effects. On the other hand, a traditional SPE clean-up will typically be the most effective and applicable when all of the analytes of interest share a common property, such as similar hydrophobicity or a shared ionic handle. Many recent modifications of these techniques have been utilized in less traditional scenarios, taking advantage of some more sensitive detectors and effectively considering the mechanistic contributors of each sample preparation step in streamlined optimizations. Coupled with automation, laboratories can increase their analyses and provide accurate and consistent results to allow for even more methods to be developed.
References
- J. Causon, D. Hanson, S. Krepich, C.T. Martha, and M. Whitmore, http://az621941.vo.msecnd.net/documents/17e5582c-83ea-4355-a194-e580274863f9.pdf
- M. Brusius and D. Spurgin, http://az621941.vo.msecnd.net/documents/56ec55c9-ddaa-4a60-be8d-cdd0ed5126a8.pdf.
- Z. Aqeel, M. Brusius, E. Chapa, J. Detsch, and S. Krepich, http://az621941.vo.msecnd.net/documents/64199b1e-0fe9-4155-8c2b-b788eb507c8a.pdf
Jenny Cybulski graduated from Vanguard University of Southern California (California, USA) with a B.S. in biology and a minor in chemistry. She now works as a product marketing manager for sample preparation products at Phenomenex, Inc. located in Torrance, California, USA.
E-mail:jennycy@phenomenex.comWebsite:www.phenomenex.com

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










