Innovations in Ion-Exchange Chromatography for mAb Variant Analysis
The Column
A surge in the development and commercialization of monoclonal antibody (mAb)-based ther-apeutics has driven the requirement for accurate and reproducible analytical methods for pro-tein characterization. Monoclonal antibodies are popular biologic drug candidates, but are sus-ceptible to a myriad of modifications during manufacture and complex degradation pathways during purification and storage, often leading to distinct charge variants that require character-ization and quantification. Ion‑exchange liquid chromatography (IEX) is a well-accepted and widely used technique to separate various mAb charge variant species for the sake of charac-terization and profiling. With the most recent advances in analytical technologies, IEX can be used to help ensure the selection of stable and efficacious mAb drug candidates, from discov-ery through manufacturing.
pixtumz88/stock.adobe.com

A surge in the development and commercialization of monoclonal antibody (mAb)-based therapeutics has driven the requirement for accurate and reproducible analytical methods for protein characterization. Monoclonal antibodies are popular biologic drug candidates, but are susceptible to a myriad of modifications during manufacture and complex degradation pathways during purification and storage, often leading to distinct charge variants that require characterization and quantification. Ionâexchange liquid chromatography (IEX) is a well-accepted and widely used technique to separate various mAb charge variant species for the sake of characterization and profiling. With the most recent advances in analytical technologies, IEX can be used to help ensure the selection of stable and efficacious mAb drug candidates, from discovery through manufacturing.
Biopharmaceuticals, or biotherapeutics, represent an extremely successful, yet complicated class of drug products. Monoclonal antibodies (mAbs) have proved to be the most exploitable modality. mAbs are highly complex biological macromolecules that have provided longstanding popular treatments for many life-threatening and chronic diseases for decades. They have a number of possible functional domains within the molecule, including binding specificity by the antigen-binding fragment (Fab) region. mAbs also have size and charge variants, and are susceptible to modifications such as glycosylation and disulfide bond formation, generating a unique profile. They may also be subject to subsequent chemical modifications, such as oxidation and deamidation, during purification and storage.
mAbs as Biotherapeutics
Regulatory bodies, such as the World Health Organization (WHO) and International Conference on Harmonization (ICH), outline the techniques recommended for biotherapeutic protein characterization. These include physicochemical characterization, biological characterization, immunochemical characterization, and the determination of product- and process-related impurities.
The guidelines state the analysis of charged variants as a regulatory requirement for biotherapeutic proteins, and give the recommended standards for the evaluation of biologics (1,2,3). The guidelines recommend the physicochemical characterization of mAb primary structure and the use of liquid chromatography (LC), including sizeâexclusion chromatography (SEC), reversed-phase chromatography, ionâexchange chromatography (IEX), and affinity chromatography. Other technologies such as capillary electrophoresis (CE) and capillary isoelectric focusing (cIEF) are also suggested.
Charge variants are often considered a critical quality attribute (CQA) in biopharmaceutical development, requiring variant-by-variant characterization of mAbs to ensure their safety and efficacy as biotherapeutics (4). Depending on the specific conditions of manufacturing or the innate properties of a mAb (such as pI), charge variant profiles can contain both acidic (lower isoelectric point species) or basic variants (higher isoelectric point species).
Because additional charge variation may occur during mAb process development and formulation, charge variant analysis is deployed across the biopharmaceutical pipeline, from discovery through to manufacturing and quality control (QC). Fast and accurate charge variant analysis can therefore speed up the development of drug candidates.
Charge Variant Analysis Methods
LC offers a robust, high-resolution approach and is widely used in biopharmaceutical analysis. IEX is a commonly used modality of LC, which separates molecules based on the difference in the ionic groups on the protein surface. Cationic exchangers bind positively charged entities, while anionic exchangers bind negatively charged entities. IEX can vary in strength depending on the pH range within which it can sustain its charge, influencing the range and type of binding that can be achieved.
CE and cIEF are complementary popular analytical techniques for charge variant characterization-their appeal primarily being their speed of analysis and ease of method development. A key advantage of IEX chromatography over CE and cIEF is the ability to collect separated species for additional analytical techniques that might give insights into structure-function relationships and tests to confirm biological significance of the charge variant. For instance, an IEX fraction can be collected and used in a ligand binding/surface plasmon resonance (SPR) study to determine if binding to the therapeutic target is preserved (5).
By comparison, it is not possible to collect adequate amounts of CE or cIEF separated species. Moreover, recent reports demonstrate that IEX chromatography using volatile eluents can be coupled to mass spectrometers for online MS detection (6) or used in a multidimensional separation for deeper characterization of a mAb (7). This is not as easily facilitated with CE or with cIEF which frequently use MS-incompatible electrolytes. Consequently, some consider IEX as being the “gold standard” for charge variant analysis.
Elution Gradients
In IEX chromatography, elution is performed using either a salt- or pH-based gradient, which elutes the bound protein by disrupting the electrostatic interactions between the protein and the stationary phase. The biopharmaceutical industry requires a precise approach for charge variant analysis, and while traditional salt-based gradients have been employed, significant method development is required to get optimal resolution of charge variants contained in different mAb samples. In the past decade, pH-based gradients have gained popularity, primarily for their more generic applicability across different mAbs. Reduced analysis cycles can also be accomplished for many samples when using pH gradients, as documented by the ability to reduce analysis time from approximately 90 down to 5–20 min (8).
The elution order of mAb variants for the two gradient types is often the same or very similar, yet pH gradients can be used to quickly obtain results without relying on prior knowledge about the sample and without having to test multiple mobile phases. An additional benefit of pH-based gradients is its facilitation of method transfer among different types of laboratories.
Innovations in IEX
A number of recent advances in chromatography have enhanced the ability to more routinely obtain high resolution mAb charge variant separations using easier to implement methods as part of modern biopharmaceutical development workflows. Using IEX columns across high performance liquid chromatography (HPLC) and ultrahighâperformance liquid chromatography (UHPLC) is a great benefit, eliminating the need to change columns and redevelop methods, and generating comparable highâresolution mAb variant separations across systems.
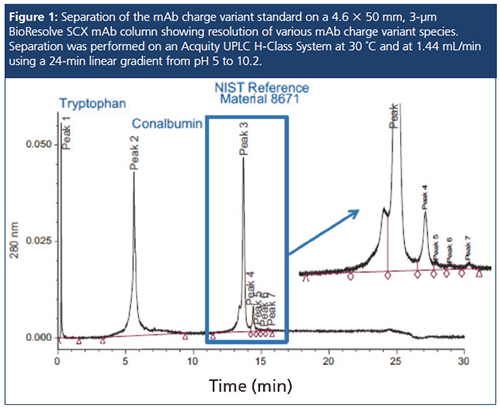
Some columns are now specifically QCâtested with a mAb charge variant standard to help ensure batch-to-batch consistency and column-to-column performance for use in validated methods. Combining advanced column technology with optimized pH buffer concentrates and mAb charge variant standards, as well as biocompatible LC systems, enables successful deployments of mAb characterization and methods, from discovery through to manufacturing. The ability to select pH, salt, or pH–salt gradients on columns with extended column life, without sacrificing separation performance, is now possible with IEX.
The Future of Charge Variant Analysis
Getting a new drug through regulatory approval can be a strenuous pursuit. In all cases, it is beneficial to have analytical assays that reliably produce accurate results.
With IEX, there is the opportunity to achieve desired high quality and reproducible data for mAb charge variant profiling. Advanced column technology, combined with HPLC and UHPLC, enables reliable identification and quantification of mAb charge variants throughout the biopharmaceutical industry, from discovery, to development, through to QC and the manufacturing process.
Method development for the LCâbased determination of biotherapeutic protein charge heterogeneity can be a timeâconsuming process because of selectivity challenges arising from minor compositional or structural differences that exist amongst protein-based drugs. Consequently, advances in instrumentation, as well as informatics and method development software, have significantly shortened the time needed to execute gradients of increasing pH, increasing ionic strength, or simultaneous increases in both pH and ionic strength. Coupled with IEX columns specifically tested to deliver reproducible mAb charge variant separations, some of these method development capabilities can be effectively used to increase the productivity of protein charge variant analyses with resulting improved workflow efficiencies.
References
- International Conference on Harmonization, ICH Q6B, Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products (ICH, Geneva, Switzerland, 1999).
- World Health Organization, Guidelines on evaluation of similar biotherapeutic products (SBPs) (Expert Committee on Biological Standardization, World Health Organization [WHO], 2009).
- World Health Organization, Guidelines on evaluation of monoclonal antibodies as similar biotherapeutic products (SBPs) (Expert Committee on Biological Standardization, World Health Organization [WHO], 2017).
- S.K. Singh, G. Narula, and A.S. Rathore, Electrophoresis37, 2338–2346 (2016).
- B. Hintersteiner, N. Lingg, P. Zhang, S. Woen, K.M. Hoi, S. Stranner, S. Wiederkum, O. Mutschlechner, M. Schuster, H. Loibner, and A. Jungbauer, mAbs8(8), 1548–1560 (2016).
- Y. Leblanc, C. Ramon, N. Bihoreau, and G. Chevreux, Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences1048, 130–139 (2017).
- C. Gstottner, D. Klemm, M. Haberger, A. Bathke, H. Wegele, C. Bell, and R. Kopf, Analytical Chemistry90(3), 2119–2125 (2018).
- K. D’Silva, K. Cook, and S. Cubbon, The Column13(9), 28–32 (2017).
Matthew Lauber is a Consulting Scientist within Chemistry R&D at Waters, where he leads an evaluation team that focuses on developing new products and methods for the analysis of biomolecules. From his graduate studies in proteomics at Indiana University, he has been applying his expertise in protein chemistry and LC–MS-based characterization methods towards the development and application of stateâof-the-art reagents and separation chemistries.
Qi Wang is a Senior Scientist in Chemistry R&D at Waters. She joined Waters in 2016 and has worked in an evaluation team on the development of new reagents and LC-based separation for biomolecules. She received her Ph.D. degree in chemistry from Brandeis University and postdoctoral training at Boston University. She has over 14 years of experience in LC–MS analysis of biomolecules, including glycans, peptides, and intact proteins.
Bill Warren has been at Waters for 32 years and is currently a Principal Product Manager involved in the development and commercialization of innovative reagents and chromatography-based technologies for the separation and analysis of proteins and synthetic DNA/RNA.
Hua Yang is a Principal Scientist at Waters Corporation and has worked there for 13 years. Hua has undertaken cutting-edge research in making advanced materials for analytical separation and sample preparation, but in more recent years, she has joined the Biopharma Group in Waters Scientific Operations to develop applications for biomolecule separations including ionâexchange chromatography.
E-mail:BioResolveManager@waters.comWebsite:www.waters.com/BioResolve

A Matrix-Matched Semiquantification Method for PFAS in AFFF-Contaminated Soil
Published: April 14th 2025 | Updated: April 14th 2025Catharina Capitain and Melanie Schüßler from the Faculty of Geosciences at the University of Tübingen, Tübingen, Germany describe a novel approach using matrix-matched semiquantification to investigate per- and polyfluoroalkyl substances (PFAS) in contaminated soil.
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.












