Mechanisms of Interaction Responsible for Alternative Selectivity of Fluorinated Stationary Phases
LCGC Europe
Fluorinated stationary phases, especially those including a pentafluorophenyl (PFP) moiety, have become popular alternatives to the more traditional alkyl (C8 and C18) phases. Many modern column lines have, in fact, been initially introduced with the standard C18 and a PFP phase because of their orthogonality. In this instalment, the differences between alkyl phases and PFP phases are discussed in terms of fundamental interactions. The origin of the interactions is also interpreted to better understand how analysts can use and control them to develop effective and rugged analytical methods.
Carmen T. Santasania1 and David S. Bell2, 1MilliporeSigma, Bellefonte, Pennsylvania, USA, 2Column Watch Editor.
Fluorinated stationary phases, especially those including a pentafluorophenyl (PFP) moiety, have become popular alternatives to the more traditional alkyl (C8 and C18) phases. Many modern column lines have, in fact, been initially introduced with the standard C18 and a PFP phase because of their orthogonality. In this instalment, the differences between alkyl phases and PFP phases are discussed in terms of fundamental interactions. The origin of the interactions is also interpreted to better understand how analysts can use and control them to develop effective and rugged analytical methods.
Stationary phases based on alkyl-bonded silica particles are most often used in reversed-phase liquid chromatography (LC) (1). High performance liquid chromatography (HPLC) columns manufactured using alternative bonded phases have, however, become increasingly popular because of the differences in selectivity and retention that they provide. Fluorinated stationary phases, especially pentafluorophenyl (PFP) moieties, have gained acceptance as alternatives to common C18 and C8 phases because of their unique selectivity (2). PFP phases have been shown to provide enhanced dipole, π-π, charge transfer, and ion-exchange interactions when compared to traditional alkyl phases (2,3).
Fluorinated silica-based stationary phases such as the one depicted in Figure 1 have shown unique retention for small, polar analytes (4–6). In particular, PFP phases generally exhibit greater retention of basic analytes than their alkyl counterparts mainly because of the presence of strong ion-exchange interactions (2,6–8). The exact origins of the ion-exchange interactions, however, remain unclear. It is important to not only understand the interactions that stationary-phase chemistry provides, but also to have some knowledge of the source of those interactions so that the appropriate controls can be incorporated into method conditions. This work is intended to provide a discussion regarding the potential origins of ion-exchange character exhibited by PFP phases. In addition, differences in the ion-exchange character of seemingly similar PFP phases are discussed. It is expected that a greater understanding of the ion-exchange interactions will result in more robust and reliable methods as well as facilitate the choice of appropriate stationary phases during method development efforts.
Background Discussion
In a recent publication, West and colleagues (9) reported on a study of ionic interactions exhibited by phenyl and PFP stationary phases in supercritical fluid chromatography (SFC). The authors noted that PFP phases exhibited greater cation-exchange properties than the phenyl phases they tested. This was attributed due to enhanced ion-exchange to the partial negative charge on the fluorine atoms in the PFP ring. The electronegativity of the fluorine atoms in the PFP ring imparts a partial negative charge on the outer edge of the ring system as well as a partial positive charge on the internal region of the ring. The ion-exchange properties may therefore stem from interactions with the ring system itself. The authors also note that the ion-exchange could possibly emanate from negatively charged silanol groups on the silica surface, but struggled to correlate the relative strength of the ion-exchange properties of various phases studied with reported carbon load data.
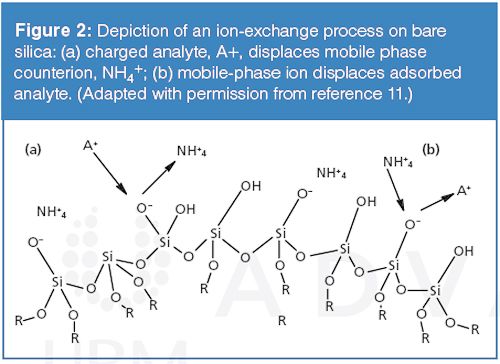
Retention in ion-exchange chromatography is governed by the exchange of the analyte ions with mobile-phase ions associated with the sorbent surface as depicted in Figure 2. The retention caused by exchange interactions is based on the relative affinities of the analyte and mobile-phase ions towards the surface. Retention is primarily controlled through manipulation of the concentration and type of mobile-phase competing ion and the charge of the analyte or surface ion through adjustments in mobile-phase pH. Most ion-exchange materials are prepared by bonding a suitable ligand such as arylsulphonic acid to a silica surface; however, silanol groups on the surface of silica-based bonded phases may also act as ion-exchangers. Although HPLC column manufacturers have made attempts to reduce the effect of silanols in reversed-phase column chemistries, it is currently considered impossible to remove all silanol groups from interacting with cationic solutes (10). It is therefore important to understand and account for ion-exchange interactions that may be present even on modern “deactivated” stationary phases (11).
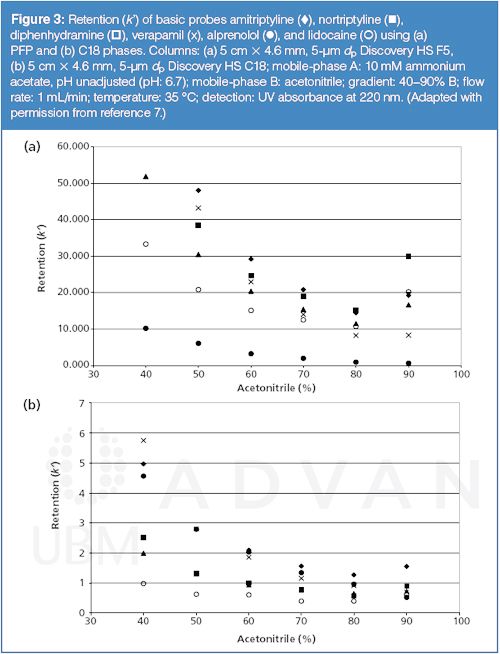
In an earlier study, Bell and Jones (7) investigated molecular interactions that contribute to “U-Shape” retention profiles on PFP phases. The authors reported that at low percentages of organic in the mobile phase, dispersive (hydrophobic) interactions dominated retention; however, as the organic percentage of the mobile phase increased, retention was dominated by ion-exchange interactions. A few key observations led to the conclusion that the ion-exchange properties were a result of surface silanol interactions rather than emanating from the PFP ligand. First, as shown in Figure 3, similar retention profile features were observed, though much attenuated, in the high organic region on a C18 phase manufactured using the same base silica as the studied PFP phase. It is in this region where the PFP exhibits dominant ion-exchange interactions. Since there is no strong dipole within the C18 ligand, the ion-exchange properties must be a result of the common silica support. Second, increasing the pH of the mobile phase from 4 to 6.7 resulted in an increase in retention for the strongly basic analytes used in the study. The pH change within this range would not be expected to alter the ionization state of a strong base (approaching 100% ionized at both pH levels), but would impact the degree of ionization of the silica surface which, for modern silica supports, exhibits an average pKa value of around 6–7 (12,13). A pH change from 4 to 6.7 would clearly result in increased ionization of the surface silanols and explain the increase in basic analyte retention. Lastly, the base silica and both the PFP and C18 bonded phases were examined by monitoring the retention of a quaternary ammonium probe as a function of mobile-phase pH. The retention profile of the probe on bare silica and the PFP phase was shown to be nearly identical, indicating that the PFP structure allows the probe nearly unrestricted access to the surface silanol groups. In contrast, the C18 phase more effectively masks the probe from the surface silanols under the conditions studied resulting in little change in retention as a function of pH.
Recent Efforts
In classification studies focused on PFP phases, including the papers by West (9)and Euerby (2), significant differences in retention and selectivity have been noted between PFP offerings from various manufacturers, especially when ionic analytes were used. Since modern bonding technologies are, for the most part, similar, these observations also point to differences in the silica surface as the main source of ionic interactions. A recent PFP product offering showed a comparison of retention for of a set of acidic, basic, and neutral probes using several commercially available PFP columns. It was noted in this comparison that the basic analytes that were clearly eluted as sharp peaks using the new PFP phase were eluted as broad peaks or were fully retained using the comparison phases. These interesting results prompted further investigation within the author’s laboratories.
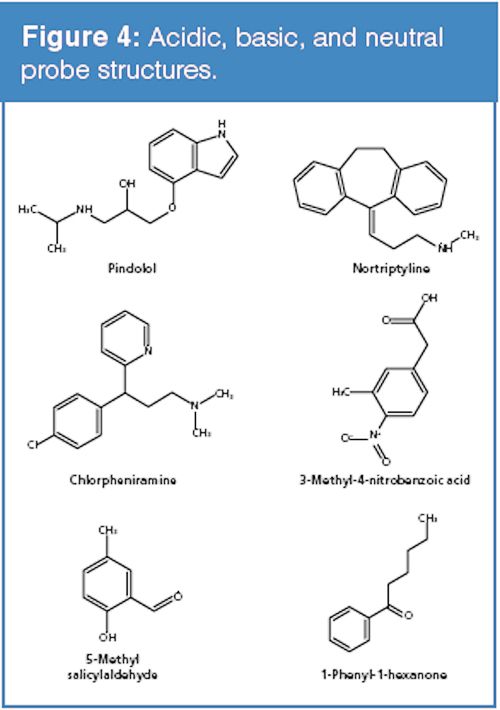
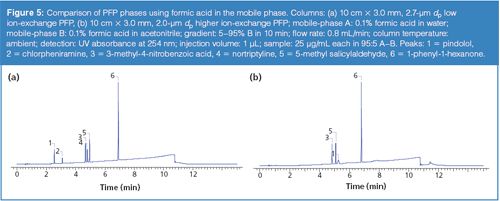
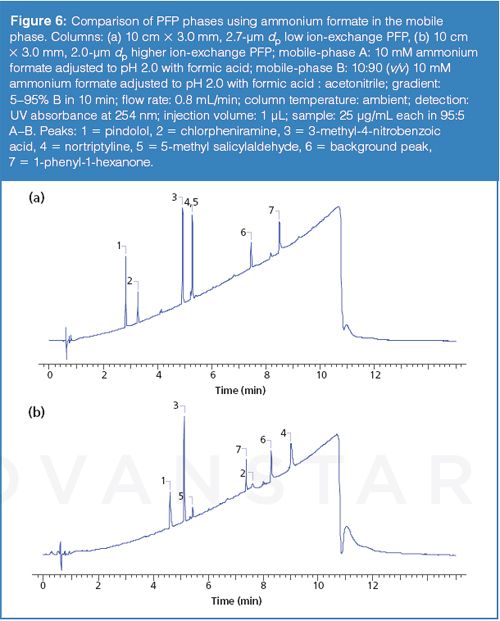
The conditions noted in the marketing material were first replicated using the “new” PFP phase and several commercially-available PFP phases. Figure 4 provides the structures of the compounds used throughout the study. The compounds 5-methyl salicyladlehyde and 1-phenyl-1-hexanone represent neutral analytes whereas pindolol, nortriptyline, and clorpheniramine are basic. The compound 3-methyl-4-nitrobenzoic acid provides an acidic component to the test mixture. The results of the initial experiment were in agreement with the findings noted in the brochure and are shown in Figure 5 (for brevity only one comparison column is presented). The data indicates that the new PFP phase exhibits less ion-exchange character than the comparison phases. The mobile-phase conditions used consisted of water and methanol acidified with formic acid (see the full conditions in the caption of Figure 5). Since ion-exchange interactions are dependent on the concentration of the competing ion in the mobile phase, a second set of experiments using 10 mM ammonium formate (pH adjusted to 2) in place of the formic acid were conducted. The chromatograms presented in Figure 6 show that the addition of the ammonium competing ion in the mobile phase for the column exhibiting greater ion-exchange character resulted in sharper and earlier eluted peaks for the basic analytes and a markedly different elution order. The elution order using the “new” PFP column remained similar. It is noteworthy to mention that low pH is often used to minimize surface silanol ionization and thus reduce undesired ionic interactions. A common misconception is that silanols are completely neutralized at low pH; however, silanols exhibit a wide range of pKa values resulting in significant ionization even at low pH levels. The rationale for why many PFP phases seem to provide increased ion-exchange character of the surface silanols relative to other bonded phases is unclear and the subject of further investigations.
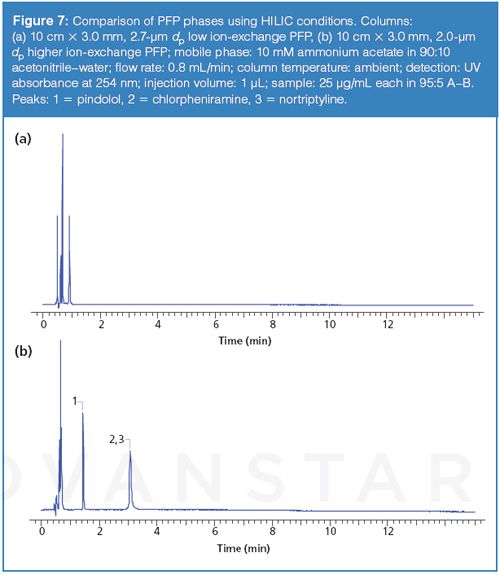
The experiments discussed above demonstrate the importance of understanding the interactions that are available from different phase chemistries and the source of the interactions. Although the new column name implies it is PFP phase, it does not exhibit the same interactions and may potentially provide very different chromatographic results. Whether this is a good or bad attribute depends on the interactions required to generate the desired separation. The lack of ion-exchange may provide more reproducible results in some cases, but may also show a lack of retention or selectivity in another. For example, PFP phases have been used in hydrophilic interaction chromatography (HILIC) where the dominant mechanism of interaction has been shown to be ion-exchange (14). Figure 7 shows a comparison of the new PFP and a competitor PFP for the same test mixture using HILIC conditions. The competitor phase, which exhibits ion-exchange character, provides greater retention and selectivity for the basic analytes in the test set and the subject PFP provides little retention because of the lack of ion-exchange interactions.
As the exact surface chemistry of the subject PFP phase is proprietary, it is speculative to state the exact reasons for the lack of ion-exchange character relative to other PFP phases. If one assumes, however, that the stationary phase is indeed dominated by PFP ligands, then this provides additional evidence that the ionic character does not emanate mainly from the ring system, but from the silica surface.
Conclusions
Pentafluorophenyl stationary phases provide alternative retention and selectivity when compared to the commonly used alkyl phases. The differences in chromatographic results stems from the fact that the PFP phases may provide interactions such as π-π, dipole, hydrogen bonding, and ionic interactions not prevalent on standard alkyl modified surfaces. When positively charged analytes are present, ion-exchange interactions often dominate. It is important, therefore, to carefully control the conditions (pH, mobile-phase ion concentrations) that ion-exchange interactions are dependent upon. In this work, the origin of the ion-exchange interaction was discussed. Although some studies have implicated the partial charges emanating from the PFP ring system as the source of the surface ion-exchange, there is strong evidence to suggest that the ionized surface silanol groups are the dominant source. It has been demonstrated that PFP phases such as the “new” column studied here provide relatively little ion-exchange interactions as compared to other PFP phases. This feature may provide more reproducible chromatographic results where ion-exchange is not desired or controlled well, but also may not provide resolution where the retention and selectivity are dependent on the presence of ion-exchange interactions. Although further study is necessary to completely understand the impact of bonding parameters such as ligand density and residual surface treatments, such as endcapping, it is clear that all PFP phases are not the same. Choosing the PFP phase that exhibits the most appropriate interactions for a given separation task should greatly facilitate the development of robust and reliable methods.
References
- D.V. McCalley, J. Sep. Sci.26, 187–200 (2003).
- M.R. Euerby, A.P. McGeown, and P. Petersson, J. Sep. Sci.26, 295–306 (2003).
- M. Reta, P.W. Carr, P.C. Sadek, and S.C. Rutan, Anal. Chem.71, 3484–3496 (1999).
- F. Pellati and S. Benvenuti, J. Pharm. Biomed. Anal.48, 254–263 (2008).
- F. Pellati and S. Benvenuti, J. Chromatogr. A1165, 58–66 (2007).
- D.S. Bell, H.M. Cramer, and A.D. Jones, J. Chromatogr. A1095, 113–118 (2005).
- D.S. Bell and A.D. Jones, J. Chromatogr. A1073, 99–109 (2005).
- H.M. Cramer, C.R. Aurand, J. McKenzie, and D.S. Bell, LCGC North Am.32, 704–712 (2014).
- C. West, E. Lemasson, S. Khater, and E. Lesellier, J. Chromatogr. A1412, 126–138 (2015).
- U.D. Neue, HPLC Columns: Theory, Technology and Practice (Wiley-VCH, Inc., New York, USA, 1997).
- D.S. Bell, “Solute Attributes and Molecular Interactions Contributing to Retention on a Fluorinated High-Performance Liquid Chromatography Stationary Phase,” (PhD thesis, The Pennsylvania State University, 2005).
- A. Méndez, E. Bosch, M. Rosés, and U.D. Neue, J. Chromatogr. A986, 33–44 (2003).
- J. Nawrocki, J. Chromatogr. A 779, 29–71 (1997).
- D. Bell, LCGC Europe28(2), 102–109 (2015).
Carmen T. Santasania is a Senior Applications Chemist at MilliporeSigma (formerly Sigma-Aldrich/Supelco) in Bellefonte, Pennsylvania, USA. He has worked at Supelco for 27 years in various areas including the Research and Development Departments, Regulatory Compliance, and Analytical Research Services. Most recently he has been working on developing LC–MS applications for small molecules. Prior to coming to Supelco, Carmen worked at Waters in Milford, Massachusetts, USA. Carmen has a BS/BA in Chemistry/Education from King’s College in Wilkes-Barre, Pennsylvania and an MS in Chemistry from Northeastern University, Boston, Massachusetts.
David S. Bell is a manager in pharmaceutical and bioanalytical research at MilliporeSigma (formerly Sigma-Aldrich/Supelco). With a B.S. degree from SUNY Plattsburgh and a PhD in Analytical Chemistry from The Pennsylvania State University, Dave spent the first decade of his career within the pharmaceutical industry performing analytical method development using various forms of chromatography and electrophoresis. During the past 15 years, working directly in the chromatography industry, Dave has focused his efforts on the design, development, and application of stationary phases for use in HPLC and hyphenated techniques. In his current role at Supelco, Dr. Bell’s main focus has been to research, publish, and present on the topic of molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. Direct correspondence about this column should go to: “Column Watch”,
LCGC Europe, Hinderton Point, Lloyd Drive, Ellesmere Port, CH65 9HQ, UK, or e-mail the editor-in-chief, Alasdair Matheson, at amatheson@advanstar.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.












