Troubleshooting Sample Preparation
An excerpt from LCGC’s e-learning tutorial on sample preparation troubleshooting at CHROMacademy.com
An excerpt from LCGC’s e-learning tutorial on sample preparation troubleshooting at CHROMacademy.com
Some of the most difficult problems to identify and solve originate from the sample preparation aspects of our work. This short article, taken from a recent Chromacademy webcast, delivers some key tips and tricks associated with troubleshooting sample filtration, an often overlooked source of potential problems.
Sample filtration, designed to remove particulate material from the sample before injection, is ubiquitous in sample preparation workflows, yet few of us pay enough attention to our filters. There are several materials of construction for both the filter body and the filter material, and the application and solvent system to be used will dictate the choice. A small range of typical filter materials is shown in Figure 1 alongside applications, solvent, and pH compatibility to indicate the importance of choosing the correct filter material.
Figure 1: Typical filter materials, suitable applications, and chemical compatibility.
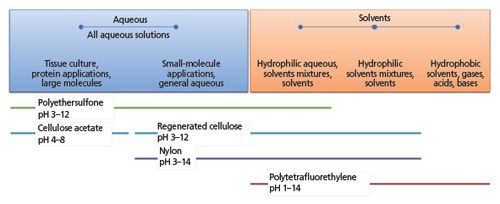
These is only a small subset of the materials available; consult your supplier for further information on the chemical compatibility of the filter materials and the suitability for various application types.
One must be sure when using organic solvents or more extreme pH levels that there will be no disintegration of the filter membrane or, as is more often the case, leaching of components into the filtrate. Any leachates may act as interferents within the chromatogram and affect both the qualitative and quantitative aspects of the analysis, especially when using mass spectrometric detectors. If there is any doubt regarding the possibility of leachates, one should rinse the filter with an aliquot (1 mL is typically enough for most syringe filter devices) to preclean the filter. This approach can dramatically clean up the resulting chromatogram in a host of applications!
Analyte adsorption (analyte binding) is a problem sometimes encountered and can severely impact the quantitative performance of the method as the degree of adsorption will vary between filters and will be affected by changes in the sample matrix. In terms of specific applications, nylon and glass fibre materials show very high bonding for proteins and peptides, and polyvinylidene fluoride (PVDF) or polyethersulphone (PES) filters are generally much more suitable. Hydrophilic membranes such as PVDF and polytetrafluoroethylene (PTFE) tend to give the lowest nonspecific binding for lower molecular weight analytes. One should always carry
out a filter binding investigation during method development by assessing
the instrument response for each analyte with a filtered and unfiltered sample (usually possible with all but the most heavily particulate laden samples).
For analytes that are very heavy in particulates, successful, facile processing requires a prefilter to avoid blockage of the lower porosity membrane. These are often called multilayer syringes and will typically allow five times more sample to pass through. Be aware that most prefilters are glass fibre, which is incompatible with protein filtration and one should identify a filter with a PVDF or PES prefilter material.
Choosing the correct filter size is a balance. Large-diameter filters allow the sample to pass quickly at lower pressure and clog less; however they risk higher levels of extractables, higher nonspecific analyte binding, and have larger hold-up volumes, which trap more sample. For samples less than 1 mL use a 4-mm filter, less than 10 mL use a 13-mm filter, less than 100 mL use a 25-mm filter, and for samples greater than 100 mL use a 30–50 mm filter. For reference, 4-mm filters have a hold-up volume of around 10 mL, whereas 30-mm filters have a hold-up volume of 60–80 mL.
It is also important to choose the correct filter porosity and for ultrahigh-pressure liquid chromatography (UHPLC) analysis, one should ensure that the pore size of the filter material is less than 2 mm.
Troubleshooting tips on a wide range of sample preparation techniques can be found in the original webcast at www.chromacademy.com/sample-preparation-troubleshooting.html
Get the full tutorial at www.CHROMacademy.com/Essentials (free until 20 March).

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.












