The Making of a Column
There are three basic types of silica glass that have been used for capillary columns: sodium, borosilicate and fused silica.
Go into nearly any gas chromatography (GC) laboratory and you will see shelves or cabinets stocked with columns; new, used, favourite and even broken ones will be on display. Yet in the midst of an abundance of columns, many chromatographers take them for granted. In the years since 1979, when fused silica became generally available to the chromatographic community as a column tubing material, the art of making capillary columns has advanced tremendously. Today's fused-silica columns, while outwardly indistinguishable from all but the earliest predecessors, deliver both general and application-specific separations with much higher mechanical stability, much lower column bleed levels, greatly enhanced inertness, and a high degree of column-to-column reproducibility.
In this instalment of "GC Connections," we'll review some of the developments in fused-silica column technology and then take a brief tour through the process of column manufacturing.
Flexible Capillary Columns
In June of 1979, I was about to finish graduate school when word came of the presentation by Dandenau and Zerenner1 at the Third Hindelang Symposium, held in April that year, in which they discussed their development of flexible capillary columns made of fused silica. Dandenau and Zerenner recounted their experiences in an LCGC article in 1990.2 As far back as 1960, the chemical advantages of silica over softer borosilicate or sodium glasses as a column material had been recognized,3 but the difficulties in working at the much higher temperatures required to soften silica for drawing out and then coiling rigid silica capillaries had inhibited much progress up to 1979.4,5 Dandenau and Zerenner's breakthrough was the successful application of optical fibre technology to the production of flexible fused-silica capillary tubing. With access to Hewlett-Packard's optical fibre facilities, where the production of step-refractive-index, silica-clad optical fibres used hollow fused-silica tubing preforms, they made the leap from flexible fibres to flexible capillaries.
I had personally drawn and coated innumerable rigid glass capillary columns in the course of completing my research, and so I was very interested in the properties of flexible fused silica; especially considering that I might have saved all those midnight hours nursing a cantankerous glass capillary drawing machine. Early in the summer of that year, a 30 m length of the new capillary tubing found its way into the lab, and as the senior GC student, I was elected to coat the tubing with a stationary phase and give it a try. I did so, using one of the chiral organosiloxane polymers from my research. I carefully cut the ends of the column with a razor blade (the "proper" cutting techniques for fused silica were completely unknown to me) and installed it in the GC inlet and detector. I set up the carrier gas pressure and split flow, lit the flame ionization detector, closed the oven door, and heated the column to about 100 °C. Upon injecting a test mixture, I was thrilled to see the expected peaks emerge and I repeated the test several times. Then I turned off the oven heater and opened the oven door, which turned out to be a fatal mistake for the column: it broke into about 50 pieces! Everywhere that the column had been in contact with its metal cage created a stress point that broke as the column cooled off.
Fortunately, I was already set to graduate and did not need any more experimental results. Less than a year later, now employed at an instrument company, I was thrust into the midst of the rush on the part of column producers to adapt their processes to the new material. I was tasked with determining the feasibility of making fused-silica columns in-house and with comparing that route to the purchase of columns from an outside supplier. At the time, there were no fused-silica column suppliers with any track record, and so the decision was not trivial, especially considering that nearly all suppliers of glass columns were engaged in essentially the same pursuit.
Fused-Silica Columns Today
Modern fused-silica columns resemble their predecessors from the 1980s in several superficial ways, while they depart from them in many significant respects. Were I to hold an early column and a recent one next to each other, I would see a long, thin silica tube coiled to about 6–8" diameter and restrained on a metal cage. The most obvious visible differences would be the colour and external appearance of the column's outer protective coating and the details of the cage itself. Not apparent to the eye would be the chemical differences in the silica material, its inner surface and the stationary phase coating.
Pure Glass
Fused silica makes a superior material for GC columns primarily because of its high level of chemical purity and not to the flexibility of the thin tubes drawn from it. The material properties of capillary fused-silica tubes are a fortuitous side-effect that has relieved those who started practising GC after 1980 of the joys of straightening and connecting coiled rigid glass capillary tubing. This was a manual procedure that required some skill. It involved heating one end of a column with a blowtorch just enough to soften the glass so that it would straighten out under the influence of gravity; at least that's how I did it. The stationary phase was destroyed, of course, so the ends had to be deactivated each time a new length was straightened. We routinely made columns an extra metre or two long in anticipation of some breakage while trying to connect them. The flexibility of fused-silica eliminated this concern, and made it easier to remove some of the column on purpose (for example, if it became contaminated with sample residue).
There are three basic types of silica glass that have been used for capillary columns: sodium, borosilicate and fused silica. Lead glass, a fourth type of silica glass, has not been used at all to my knowledge. All of these glasses consist primarily of SiO2, but there are significant differences. Sodium and borosilicate glasses are the easiest to form and work with. Sodium (also called soda-lime) glasses have the lowest working points at around 1200 °C. Borosilicate glass, which is used for laboratory glassware, is workable at around 1600 °C. Their relatively low working temperatures are obtained by adding significant quantities of sodium and calcium, or boron, respectively, and they contain about 70–80% silica. Other impurities are present at the per cent or lower levels. For capillary columns, the impurities cause peak distortion, tailing and sometimes partial or complete loss through active reversible or irreversible adsorption of many of the polar, polarizable and aromatic compounds commonly separated by GC. These adsorptive effects are more significant at trace levels of the analytes. A tremendous amount of effort had been expended towards deactivating glass capillary columns before 1979 that resulted in innumerable methods to remove, replace or mask the active sites. I had employed some of these in my graduate research on borosilicate glass with varying degrees of success.
As had been recognized early by Desty,3,4 pure fused silica does not cause the undesired effects of impurities in softer glass. However, as researchers began to work with the new material, they quickly discovered that fused silica was definitely not perfect, and many of the surface preparation techniques used for earlier glass columns did not necessarily work with fused silica. To confuse matters, some early fused silica was produced from highly purified quartz sand while other material was synthetically produced through the hydrolysis of silicon tetrachloride. The former is known as fused quartz and the latter as synthetic fused silica. Fused quartz tends to contain low but significant impurity levels, and it was soon abandoned as a capillary GC column material. Synthetic fused silica contains less than 1 ppm of metallic impurities, while good fused quartz can include around 20–30 ppm metallic impurities. Both fused quartz and synthetic fused silica have very low coefficients of thermal expansion, so they tolerate temperature extremes well.
The glass drawing process (see Figure 1) feeds a relatively large preform tube into a furnace that heats the glass to its flowing point. The inside of the tubing and the furnace cavity are continuously purged with inert gas to avoid oxidation and prevent the influx of ambient atmospheric contaminants. The glass is drawn out more rapidly than the incoming preform feed rate, which produces a smaller and longer tube with reduced cross-sectional dimensions. The draw rate ratios used for fused-silica material typically yield several thousand metres of finished tubing for each metre of preform that is fed into the furnace. A laser optical gauge constantly measures the outgoing tubing diameter and provides feedback to the rate controllers: the tubing dimensions are controlled very tightly as a result. Immediately after passing the laser gauge, the tubing enters multiple stages of external coating application and curing, and is tested for tensile strength. Finally, it is wound up on large spools, sealed and stored prior to being coated internally with stationary phase.

Figure 1: Fused-silica capillary tubing drawing tower schematic. The preform feed mechanism (1) feeds a fused-silica preform (2) into a 2000 °C furnace (3) at a slow rate. The tubing softens in the furnace and is drawn out at a higher speed. A laser gauge (4) measures the tubing size and adjusts the rates to maintain a constant diameter. The drawn tubing is externally coated with a protective coating (5) and the coating is cured (6). A draw capstan (7) controls the rate at which the tubing is drawn. After testing for tensile strength (8), the tubing is wound on a spool (9) for storage. This diagram is not to scale. Figure used with permission of Polymicro Technologies, Phoenix, Arizona, USA.
The outer surface of the tubing is very sensitive to physical stress. The slightest imperfection in the silica will propagate into a major crack and cause breakage under any significant strain. The silica preforms are carefully fire-polished before the tubing is drawn to ensure a defect-free result. Bending fused-silica tubing produces high stress levels that the tubing tolerates because of its very high tensile strength.
Immunity to external abrasion is achieved by coating the outside of the column immediately after it is drawn. Several different types of protective coating have been employed for fused-silica columns, including silicone, polyimide and aluminium. Of these, the latter two are still in use. The main limitation of silicone coatings is their relatively low temperature limit, which conventional GC analyses can exceed easily. The vast majority of fused-silica columns today are coated on the outside with multiple layers of the familiar brown to yellow polyimides. Polyimide coatings, which originated in the aerospace industry, can tolerate temperatures of up to about 400 °C for brief periods, and of course aluminium has a much higher limit. The multiple column breaks that I and many others encountered early on have not been seen in new columns for more than two decades, because of improvements in external coating materials and processes.
The process of drawing fused-silica capillary tubing subjects the material to temperatures close to 2000 °C, effectively removing all surface water and leaving Si-O-Si bond bridges and some Si-OH functionality on the inner and outer surfaces. This initially pristine surface would rapidly take up atmospheric water upon cooling and during subsequent storage and handling if the tubing were not purged with inert gas. The amount of water and how it is attached to the inner surface plays a crucial role in deactivation and stationary phase chemistry. The level of bulk hydroxyl groups in the material has important effects on its optical properties that are not of much concern to gas chromatographers but may be of interest in liquid chromatography systems that use the column end, stripped of the external coating, as an optical window to the mobile phase.
Inside Knowledge
The raw inner surface of freshly drawn fused-silica capillary tubing contains very little water. However, the surface Si-O-Si bridges are strained and will readily absorb water from the atmosphere or from solvents and solutions used in the deactivation and coating processes. Additional water can be adsorbed on the surface as well. Controlling the amount and type of -OH functionality on the inner surface — whether as reversibly adsorbed water molecules or as terminal silanol (Si-OH) groups — is vital to successfully engineering the desired stationary phase coating, not only in terms of its retention characteristics, but also for producing desired levels of residual chemical activity, bleed levels and coating stability. The reagents used for surface treatment and coating must themselves be highly purified because the fused-silica surface will easily adsorb contaminants, including trace metals, from them as well.
The task of coating the inside of a fused-silica capillary with stationary phase is a multistep process consisting of pretreatment and deactivation, coating and final polymerization, conditioning and testing. Each class of stationary phase coating — non-polar siloxanes, moderately polar siloxanes, highly polar siloxanes, carbowaxes and others — requires different treatments that can also depend on the film thickness, column length and inner diameter, upper temperature limit and application scope.
Pretreatment and Deactivation
The purpose of these initial steps is to create a consistent surface that is ready to be coated with the stationary phase, while also ensuring that the surface is suitably deactivated. In the finished column, chemical activity is measured relative to one or more specific compounds, including acidic, basic and aromatic types. Capillary column test mixtures contain compounds such as octanol, dimethylphenol, dimethylaniline and naphthalene that exhibit peak tailing, peak area loss or retention shifts on columns that lack suitable deactivation, have exposed silica surfaces, or are contaminated.
It is difficult to balance residual chemical activity to achieve high performance for all compounds: some treatments produce better results with acidic compounds, while others are better for basic compounds. In general, however, fused-silica material with significant levels of surface silanol groups tends to be acidic and requires different treatments when basic compounds are the target analytes; many capillary columns are specifically tailored for basic or acidic compounds. In any case, appropriate expectations for test mixture results should be determined according to the specific application.
Excess surface activity, as well as the presence of impurities on the surface can also lead to higher column bleed levels. Column bleed refers to the increased rate of release of material from the stationary phase and inner surfaces as column temperatures rise. Bleed is observed as a rising baseline profile that obscures small peaks and adds noise to the detector signal, and eventually as reduced retention times and increased solute activity as a result of bulk loss of the stationary phase. One source of column bleed is the breakdown of the stationary phase polymer under the influence of active chemical groups on the surface. Bleed also occurs in the presence of introduced impurities from sample injections as well as from the action of free oxygen in the carrier gas. Producing low bleed columns requires careful attention to reagent chemistry and purity at all stages of column production. Silica surface activity is masked to some extent by thicker stationary film coatings, but at the same time, thicker coatings tend to exhibit lower efficiencies and higher bleed levels themselves.
Coating and Polymerization
The initial stationary film must form a smooth stable layer upon coating inside the column. Its stability depends upon the surface tension of the treated inner silica surface, as well as some other variables that are described later, and so any pretreatments must leave the silica surface in an appropriate state of readiness to accept the coating. In the instance of some bonded stationary phases, the coating material chemically bonds with surface silanol or siloxane groups or with other intermediary reactive groups introduced as part of the initial preparatory treatments. Here, the term "bonded phase" means that there are chemical bonds between the bulk of the stationary phase polymer mass and the surface of the column. In theory, a column with the stationary phase chemically bonded to the tubing will exhibit better mechanical and thermal stability. However, in the face of external destabilizing influences such as free oxygen or sample impurities, surface bonding can give limited improvement. Thus, the importance of operating and storing the column clean and oxygen free cannot be overemphasized.
A stationary phase film or a layer of pre-polymer reactant solution is deposited onto the column inner walls in one of two ways, either by dynamic or by static coating. Figure 2 illustrates these two techniques. In dynamic coating, a plug of solution passes through the column at a (hopefully) controlled rate, under positive gas pressure. The trailing meniscus leaves behind a layer of solution the thickness of which depends upon the speed at which the plug moves as well as the surface tension and wettability of the solution on the column inner surface. After the excess solution has left the column, continued gas flow evaporates the solvent and leaves the dissolved material behind. The dynamic coating method is difficult to control because the plug grows shorter as it sheds liquid and therefore, the pressure required for constant plug velocity decreases during the process. In my hands, the plug velocity would go up exponentially as it got near the end of the column, which always ruined the last few metres of the column. I simply cut that last section off after the column was finished, but I was never very satisfied with this method.
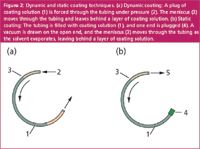
Figure 2: Dynamic and static coating techniques. (a) Dynamic coating: A plug of coating solution (1) is forced through the tubing under pressure (2). The meniscus (3) moves through the tubing and leaves behind a layer of coating solution. (b) Static coating: The tubing is filled with coating solution (1), and one end is plugged (4). A vacuum is drawn on the open end, and the meniscus (3) moves through the tubing as the solvent evaporates, leaving behind a layer of coating solution.
In static coating, the entire capillary tube is filled with a solution of the stationary phase or its precursors. One end is carefully capped without a gas bubble and then a vacuum is drawn on the opposite open end. I remember the anticipation at the moment of first applying vacuum to a new column and waiting to see if a bubble would form in the solution. If you were quick enough to spot it then the column could be recovered, otherwise it had to be drained and rinsed out before starting over.
Once started, the solvent evaporates under the influence of the vacuum and leaves the dissolved material behind: as the solvent evaporates, the meniscus moves back along the column towards the closed end. The rate of static coating also varies during the process and the speed of evaporation slows down as the meniscus progresses along the length of the tubing. However, in this instance, the concentration of the solution is what determines the amount left behind and not the evaporation rate. The main trick with static coating is to control the tubing temperature well; the liquid in the tubing acts like a very sensitive thermometer and small temperature variations can cause the meniscus to fluctuate and deposit an uneven layer. In my limited experience, the static technique gave the best results and I was often able to make columns that performed at close to theoretical efficiency.
All of the columns I made were coated with a solution of the stationary phase in its final state: once the solvent evaporated, the column was done and ready for conditioning. I worked with fairly low molecular weight polymers that I had synthesized and I was not concerned with temperature limits. To obtain columns that are useful at higher temperatures, the polymer molecular weights become much higher and the polymer structures more highly cross-linked. Such large polymers have high viscosities and are more like gums than liquids. Even when in solution, if they can be dissolved, they are difficult if not impossible to force to fill an entire capillary tube for static coating at concentrations that deposit enough material for the desired phase ratio.
Instead, the tube is filled with a solution of oligomers or other precursors of the final material plus polymerization initiators. The polymerization reaction is performed in situ, either before or after the solvent is evaporated, leaving the newly formed stationary phase material behind. The polymerization chemistry is chosen so that only volatile by-products are produced, which can be purged out of the column during initial conditioning.
Conditioning and Testing
After coating and initial drying, the new column is conditioned by gradually programming its temperature to the upper limit with highly purified carrier gas flowing. The temperature is then held there long enough to vaporize and remove residues that otherwise would be seen as column bleed later on. After an initially high impulse of exiting residues, primarily the leftovers from coating and polymerization, the background level decreases rapidly until it reaches a nearly steady value. This will be the initial bleed level of the new column. The bleed level will tend to decrease gradually over time in the absence of external influences like trace oxygen or chemical contamination, but it cannot be expected to go to zero. The amount of bleed emitted from modern low-bleed columns is remarkably small when the subnanogram detection capabilities of many GC detectors are taken into consideration. This is both a benefit to gas chromatographers and a challenge to column manufacturers who must strive to meet end-users' expectations.
Finally, after conditioning is complete, the column is tested for chromatographic performance with a test mixture that is appropriate to the intended application. There are many different test mixtures available from chromatography suppliers. In addition to the fundamental polarity test compounds mentioned earlier in this article, hydrocarbons, fatty acids and their methyl esters, polyaromatic hydrocarbons (PAH), and alcohols, plus common herbicides and pesticides are all available in various general purpose test mixtures. Many analytical protocols call for the use of a standardized mixture of the actual target compounds to test columns and this is certainly a good idea. However, the occasional use of a general column test mixture can often reveal developing problems before they surface in the day-to-day methodology. Before putting a new column into routine service, I like to test it with the same or a similar test mixture as was used by the column manufacturer. These mixtures usually cover a broader range of polarities and can constitute a more stringent test. Later on, periodic tests with the same column test mixture can be compared with the original test that was performed at installation, which may reveal a developing problem.
Conclusion
Although very similar in appearance to the earliest fused-silica columns of the 1980s, modern fused-silica columns and the tubing they are made of have undergone decades of development and improvement. The process of making capillary GC columns has evolved from something that a graduate student could perform starting right from drawing out the tubing to a technically sophisticated procedure that involves precision machinery, sophisticated polymer chemistry and highly trained technical specialists. As far as I know, all of the old soft-glass drawing machines have been retired or put into museums.
References
1. R.D. Dandenau and E.H. Zerenner, J. High Res. Chromatogr., 2, 351–356 (1979).
2. R.D. Dandenau and E.H. Zerenner, LCGC, 8 (12), 908–912 (1990).
3. D.H. Desty, J.N. Haresnape and B.H.F. Whyman, British patent 899,909 (applied 9 April 1959, issued 27 June 1962).
4. D.H. Desty, Chromatographia, 8, 452–455 (1975).
5. K. Grob and G. Grob, Wissenschaftl. Zeitschr. Karl-Marx-Univ., 26(4), 379–384 (1977).
"GC Connections" editor John V. Hinshaw is senior staff engineer at Severon Corp., Hillsboro, Oregon, USA and a member of the Editorial Advisory Board of LCGC Europe.
Direct correspondence about this column to "GC Connections," LCGC Europe, Advanstar House, Park West, Sealand Road, Chester CH1 4RN, UK, e-mail: dhills@advanstar.com
For an on-going discussion of GC issues with John Hinshaw and other chromatographers, visit the Chromatography Forum discussion group at http://www.chromforum.com
Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.












