On-line Supercritical Fluid Extraction-Capillary Gas Chromatography for the Analysis of Diesel
LCGC Europe
Direct coupling of SFE with GC has advantages over the off-line alternative.
Introduction
For the analysis of analytes with sufficiently high vapour pressures and thermal stabilities, capillary gas chromatography (GC) is the method of choice because of its very high resolution per unit time in comparison with other chromatographic techniques. Another advantage of using GC is the wide variety of detectors that are available.
As the most commonly used supercritical fluids (e.g., CO2, N2O, SF6) are gases at ambient conditions, SFE can also be coupled directly with capillary GC using simple techniques.
Direct coupling of SFE with GC has advantages over the off-line alternative. First, SFE–GC eliminates all sample handling and associated errors between extraction and chromatographic analysis. Second, SFE–GC can yield maximum sensitivity because 100% of every extracted species can be transferred onto the GC column. Consequently, the sample volume can be minimized because the whole extract is analysed. Third, analysis time is shorter with SFE–GC and automation is easy to perform.
There are three basic steps to perform successful SFE–GC:
1. Extraction of analytes into the supercritical fluid
2. Depressurization of the supercritical fluid, followed by trapping and focusing of the extracted analytes
3. GC separation of the target analytes.
For good chromatographic resolution it is important during SFE–GC to focus and collect the extracted analytes at the head of the chromatographic system during the extraction step. The major problem to be solved when quantitatively trapping and focusing analytes from the SFE effluent stems from the high gas flows encountered upon depressurization.
Refocusing is vital to obtain good chromatographic peak shapes and this has been achieved in one of two ways: using the analytical column itself1–7 or a retention gap for collection of analytes,8–11 or collecting the analytes in an external accumulation device.12
To our knowledge, only one paper has reported on an SFE–cold trap–GC–MS. The obvious problem with collecting directly onto the column is that the main part of the expanded gas flows through the analytical column. As a result, use of some detectors may be restricted because of interferences between some gases and the detector (e.g., N2O and an ECD).8 In these instances purging with carrier gas after extraction is necessary. Another problem is burdening the GC column with large amounts of effluent.
For external collections, analytes are depressurized into a cold trap or sorbent resin.8 After extraction, the cold trap is heated and the analytes are transferred to the GC column by the carrier gas. These methods have the advantage that depressurized extraction fluid does not flow over the analytical column and, consequently, the effect of the fluid on the detector can be ignored. However, one disadvantage of empty cold traps is loss of volatile compounds during the extraction step.13 Sorbent traps (e.g., Tenax) that can be thermally desorbed for analyte recovery may be more promising.13
Coupling SFE to GC can be achieved through various techniques. One technique involves inserting the extraction cell outlet restrictor directly into a conventional GC split injection port,5 or by inserting the restrictor into the capillary column through a conventional on-column injection port.13
The technique developed in this article uses a cold-trap system. The extraction cell outlet restrictor is inserted into the cooled glass liner through a direct interface with the carrier gas connection.
On-line SFE–GC will also be compared with thermal desorption. The analysis of diesel fuel thermally desorbed from Tenax yielded, after optimization, good recoveries for compounds in the range of dodecane (C12) to octadecane (C18).14 However, for molecular weights outside this range recoveries were not satisfactory.
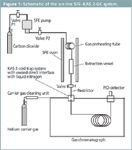
Figure 1: Schematic of the on-line SFEâKAS 3-GC system.
Experimental
Apparatus: A schematic of the SFE–GC system is shown in Figure 1. All GC analyses were performed on a 5890 Series II gas chromatograph (Agilent Technologies, Waldbronn, Germany) equipped with a flame ionization detector. Helium was used as the carrier gas, and a head pressure of 10 psi was set. The helium purity was 7.0, after purifying with a MegaSorb gas purifier system (Messer-Grießheim, Siegen, Germany). The GC was equipped with a KAS 3 cold trap system (Gerstel GmbH, Mülheim, Germany) to cryofocus the analytes in the glass liner of the injection system. The septum-less injector of the cold trapping system was replaced with a direct interface to the carrier gas connection (Figure 2). A cross-linked methyl-silicone fused-silica capillary column [DB1, 50 m × 0.32 mm i.d., film thickness 1 μm (SGE, Weiterstadt, Germany)] was used for all GC analyses. The GC instrument conditions were as follows: temperature, 40 °C; hold for 5 min; ramp at 20 °C/min to 300 °C; hold for 20 min.
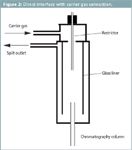
Figure 2: Direct interface with carrier gas connection.
The supercritical fluid extractions were performed using an SFX-100 pump (in-house made, Siegen University). The carbon dioxide was technical grade (Messer-Grießheim). The extraction cell was an empty HPLC column (40 × 4 mm i.d., stainless steel) inserted into and temperature-controlled by a TDS1 thermal desorption oven (Gerstel).
The SFE conditions were as follows: pressure, 40 MPa; temperature, 100 °C; time, 30 min; approximately 18 mL liquid CO2. Pressure was maintained by a linear fused-silica restrictor [25 cm × 25 μm i.d. (SGE, Weiterstadt)]. During SFE extraction, the split ratio was set to maximum (300 mL/min) to allow ventilation of the gas from the injector. The restrictor was inserted directly into the cryo-cooled glass liner of the cold trap system.
Thermal desorption experiments were performed with the TDS1 unit (Gerstel).
Chemicals: A characterized standard diesel fuel (Table 1) was dissolved in nanograde n-hexane (Promochem, Wesel, Germany). The concentration was 18.67 μg diesel fuel in 1 μL n-hexane (16.11 μg carbon/μL n-hexane).
Procedure: The extraction vessel was filled with 110 mg of Tenax TA (Dräger, Lübeck, Germany). The Tenax had been cleaned before use for one hour with a flow of purified helium at 280 °C. The Tenax was spiked with 1 μL of an n-hexane solution containing 18.67 μg standard diesel fuel. The extraction vessel was then closed and mounted in the SFE–GC system. The restrictor was inserted into the cold trap system and sealed with a vespel ferrule. The cold-trap system was then cooled to the desired trapping/focusing temperature of –50 °C.

Table 1: Properties of standard diesel fuel density.
The extraction vessel was pressurized to 40 MPa and heated to 100 °C. Valve 1 was closed (see Figure 1). Extraction was performed with the interface split valve open (split ratio of 300:1). The extracted components were deposited in the cold-trap, while the depressurized gaseous carbon dioxide was vented through the split valve.
After 30 min the extraction was stopped and the outlet valve of the extraction vessel was closed. The restrictor was left in the injection port. The cold-trap system containing the cryofocused analytes was rapidly heated using a temperature ramp of 10 °C/s up to 310 °C and held at this temperature for 5 min. The components were then transferred to the capillary GC column. The total extraction took less than 35 min.
During GC separation the extraction vessel was decompressed and the next extraction vessel was mounted in the system.
Results and Discussion
As described previously, recoveries of certain compounds are very dependent on the trapping conditions chosen for the KAS.14 For thermal desorption of our samples, the TD–KAS system was optimized for good recoveries in the C12–C18 range. Because these compounds are neither gaseous nor particulate, automobile engineers call them semivolatiles. Our previous results from thermodesorption cold trapping injection GC served as a base for SFE–KAS–GC on-line injection.
The spiked Tenax samples were analysed as described earlier. Recoveries were calculated relative to the results obtained with liquid GC injections of the same sample. Recoveries calculated for on-line SFE–GC were based on those obtained by direct liquid injection. Calculations were always based on peak areas as obtained from the FID signal. The hexane-diluted diesel fuel was manually injected resulting in relatively high standard deviations. These cannot be neglected, when looking at the correspondingly higher RSDs for SFE–GC. The reproducibility of GC and SFE was determined by performing five experiments on different days. Trapping conditions in the KAS were optimized for > 80% recoveries in the dodecane to octadecane range, again to be comparable with data from thermal desorption. Trapping temperature, time, flow and needle length had to be adjusted. The results are shown in Table 2.

Table 2: Comparison of analyte transfer into GC.
The average recovery for all individual components with on-line SFE–GC was 80%. In the range from dodecane to octadecane recoveries were in the order of 80–100%. A second extraction of the same Tenax trap under exactly the same conditions increased the recovery by only 2–5%. It appears that thermal desorption KAS had an obvious cut off above the C18 alkane. In fact, even the C18 alkane had a lower recovery than that for on-line SFE–KAS–GC. Up to the C24 alkane, the upper end of the investigated range, recoveries were above 60% when compared with liquid injection. This, however, is far superior to TD–KAS–GC. Compounds above C24 were not studied here, because in diesel emissions, higher molecular weight compounds are adsorbed onto particles. The most volatile compounds, for example, n-decane, may be partly lost because of breakthrough in the cold-trap.
Considering all the facts the reproducibility of 15–22% was acceptable for a matrix such as diesel fuel. For the more volatile compounds reproducibility was 25–57%. A typical chromatogram for a liquid injection of diesel fuel is shown in Figure 3(a). A chromatogram from the SFE–GC procedure with the same sample is shown in Figure 3(b). TD–KAS is acceptable only in the range from undecane with 81% recovery to octadecane with 64% recovery. For decane it is comparable to on-line SFE–GC although not satisfactory. For alkanes above nonadecane thermal desorption cannot be used.

Figure 3: FID chromatogram of a standard-diesel fuel, (a) liquid injection and (b) SFEâGC on-line.
Conclusions
The on-line SFE–KAS–GC system works reliably as described and extractions are easy to perform. Because CO2 does not enter the GC column it does not restrict the choice of detector or column. The system could be used for different matrices and environmental samples, for example, exhaust gas analysis. As an example the analysis of diesel fuel is given and recoveries were satisfactory. Recoveries were superior, considering the extended range (C11–C24 alkanes), when compared with thermal desorption (C11–C18 alkanes). SFE–GC is a good alternative method to thermal desorption for semivolatile compounds or thermally unstable analytes. The recoveries for more volatile compounds can be increased by lowering the KAS cold-trap temperature.
We are currently performing further investigations into the use of on-line SFE–KAS–GC for the analysis of diesel exhaust fumes trapped on Tenax.
Bernd W. Wenclawiak studied Chemistry at the University of Münster (Germany), became Dr. rer. nat. in 1978, finished his Habilitation (Analytical Chemistry) in 1986, became associate professor at the University of Toledo from 1984–1988 and has been professor of Analytical Chemistry at the University of Siegen since 1989 and dean since 2001. Heiko M. Herrmann studied Chemistry at the University of Siegen (Germany) and became Dr. rer. nat. (analytical chemistry) in 1997. He has been a product specialist for HPLC and LC–MS with Dionex GmbH Germany since 2000.
References
1. S.B. Hawthorne, M.S. Krieger and D.J. Miller, Anal. Chem., 60, 472–477 (1988).
2. S.B. Hawthorne, M.S. Krieger and D.J. Miller, Fresenius Z. Anal. Chem., 330, 211–215 (1988).
3. K.J. Hansen, Anal. Chem., 67, 3541–3549 (1995).
4. M. Lohleit and K. Bächmann, J. Chromatogr., 505, 227–235 (1990).
5. J.M. Levy, J.P. Guzowski and W.E. Huhak, J. High Resolut. Chromatogr. Chromatogr. Commun., 10, 337–341 (1987).
6. B.W. Wright et al., Anal. Chem., 59, 640–644 (1987).
7. S.B. Hawthorne, D.J. Miller and J.J. Langenfeld, J. Chromatogr. Sci., 28, 2–8 (1990).
8. M.W.F. Nielen et al., J. Chromatogr., 474, 388–395 (1989).
9. H.R. Johansen, G. Becher and T. Greibrokk, Anal. Chem., 66, 4068–4073 (1994).
10. E.S. Francis et al., J. Microcol. Sep., 7, 23–28 (1995).
11. Z. Liu, P.B. Farnsworth and M.L. Lee, J. Microcol. Sep., 4, 199–208 (1992).
12. R. Fuoco et al., Anal. Chim. Acta, 346, 81–86 (1997).
13. S.B. Hawthorne, Coupled Supercritical Fluid Extraction-Capillary Gas Chromatography (SFE-GC), B.W. Wenclawiak Ed., Analysis with Supercritical Fluids: Extraction and Chromatography, (Springer-Verlag, Berlin, 1992), 61–72.
14. R.H. Hammerle et al., SAE Technical Paper 62, 952353 (1995).
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.










