LC-30 A Nexera — UHPLC in practical use
Shimadzu Application Note
Dr Bjoern-Thoralf Erxleben, Shimadzu Europa GmbH, Duisburg, Germany.
Based on genetic factors, we are initially skeptical of all things new. This is not different with UHPLC. "UHPLC is nothing new", or "all UHPLC systems are the same" are some of the preconceived beliefs. Why, then, is each new UHPLC system being put to the test over and over again? And why are users often surprised that not everything is what it seems, or that separations are actually improving? What will Shimadzu's Nexera system be submitted to, what needs to be tested and what will be the outcome? Each test is different. The main focus is essentially determined by the objectives. In part, existing standard operating procedures are applied while departments and working groups can be engaged to perform comprehensive tests. Practical aspects (i.e., real separation examples and chromatographic conditions occurring in daily routine) commonly have priority, as it has been shown that testing of specific gradient profiles are only of limited significance for later routine use. To generalize on testing to date and in the future, as well as how these tests are being conducted, is difficult. The following outline provides the best overview.
Flow and Pressure Range
A common test increases the flow rate while checking whether the retention times in the chromatogram are proportionally reduced. This approach is not necessarily the method of choice for fast LC — the crucial question being, how much the flow rate can be increased before reaching the pressure limit? Conversely, doubling or tripling the column length while using the same column material is possible, which considerably increases the plate number and thereby the potential chromatographic resolution. The example in Figure 1 shows the separation of a compound mixture at different flow rates.
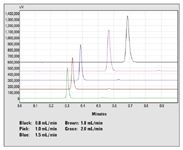
Figure 1: Compound mixture at different flow rates.
Mixing Behaviour
The system volume plays an important role in UHPLC applications. Small mixing volumes with the best possible mixing of the mobile phase components are, therefore, a first step. However, the need for larger mixing volume with complex mixtures or addition of ion-pairing reagents needs to be considered.

Figure 2: Overlay of six chromatograms.
Gradients
A further test is related to gradient accuracy and reproducibility. For this purpose, long and flat gradients are often used for simultaneous separation of complex mixtures (e.g., tryptic digest of a protein). Many labs often perform such tests using standard mixtures, which, in addition to obtaining results for a particular instrument, also enable comparison between instruments. Subsequent overlay of the chromatograms of retention times and (or) peak areas shows what a UHPLC system can achieve.
Sample Introduction Linearity and Sample Carryover
Nexera focuses much attention on the autosampler and intensive testing. Sample carryover, speed and linearity are specifications that critically influence results of the UHPLC system and are often regarded as 'killer criteria'. It is, therefore, not surprising that carryover tests are conducted under complex conditions. Figure 3 shows the results of a carryover test for two tricyclic antidepressants (amitryptiline and desipramine) with the LCMS-2020 used as detector, where further rinsing options were gradually implemented in addition to the standard rinsing procedure. The example shows that by already implementing part of the extensive rinsing options, carryover can no longer be detected via MS.

Figure 3: Carryover test and results.
Fast injection is particularly important for ultrafast separations and very high sample capacity. In addition to the speed of injection, the cycle time — usually measured from the 'start' command of the chromatographic run to the next injection — is essential, as this involves not only the absolute injection speed, but also subsequent rinsing steps and associated valve switching and needle movement. Newly implemented functions, for instance overlapping injection, reduce the time per analysis cycle, which can lead to considerable savings in time. These types of examples represent the quality of the injector as well as the stability of the separation column and the reproducibility of the results.

Figure 4: Example - overlapping injection and its effect on the total analysis time.
Detection
In addition to stable pressure, efficient mixing and fast, precise and carryover free injection, all of the tests conducted require highly sensitive detection. To make this possible, data acquisition of up to 100 Hz for standard detectors (UV, PDA and fluorescence) and a tempered flow cell to ensure low noise, are fundamental parameters. Also the LCMS-2020 proves to be a highly sensitive and highly suitable UHPLC detector.

Shimadzu Europa GmbH
Albert-Hahn-Str. 6-10, D-47269 Duisburg, Germany
tel. +49 203 76 87 0 fax +49 203 76 66 25
E-mail: shimadzu@shimadzu.eu
Website: www.shimadzu.eu

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.




















