Kurt Grob — One of the Fathers of Capillary GC
LCGC North America
In this month's installment, Kurt Grob's life and achievements are described by someone who knew him like few other people did: his son, Koni Grob.
I believe that biographies of outstanding persons should not only deal with the achievements, but also with the environment and style of their working, as we might learn from this. What impressed me most about my father's work is that he played a major role in developing the art and science of capillary gas chromatography (GC), and that he did this virtually alone as a hobby in his spare time — his real job was teaching. His productivity was far beyond the norm, and this is the focus of my conclusions.
Teacher of Chemistry
Kurt Grob was born in 1920 in the German-speaking part of Switzerland. His father was a teacher and his mother instructed sewing and knitting. For many years, he intended to study (Protestant) theology, but a telephone conversation with a chemistry professor he had never met convinced him that studying chemistry would be a better choice. This was during World War II, and even though Switzerland escaped direct participation in the war, he spent more time in trenches at the border than in the lecture halls or laboratories. His doctoral thesis at the Swiss Federal Institute of Technology (ETH), Zürich, was on tobacco fermentation. In 1948, he married a sewing teacher and soon began teaching elementary chemistry to 16–19 year-old students at the Gymnasium (college) of Zurich. He maintained this position up until the age of 62.
He must have been an excellent teacher and motivated many students to study chemistry. Without having received an education as a teacher (it was him introducing didactics in chemistry at the University of Zurich), he passionately experimented with the ways a subject could be presented in a manner that would stimulate his students and give them a real understanding (Figure 1). During exams, students were allowed to bring along all notes and books they wanted: application of the new knowledge was the scope, not rote repetition — which was probably tough for the less-gifted students.

Figure 1
It was one of his basic philosophies that a good chemistry teacher should work in chemistry himself to provide a lively image of this field to his students — and he did this throughout his professional life. Soon he started teaching other teachers by organizing workshops in the chemical industry, which were primarily hands-on, to replace the missing contact with real world chemistry. In 1973, he received his first honorary doctorate for his achievements in teaching chemistry.
Tobacco and Cigarette Smoke
In the first year after completing his own studies (1948), he designed a plant for fermenting tobacco in the Italian-speaking south of Switzerland. It was only after this that he began teaching, but during school vacations in spring, summer, and autumn he continued to work at the fermentation plant, which meant that our family followed him to the Ticino on these "vacations."
Around 1958, the health hazards of tobacco smoke became abundantly clear. In the spirit of that time, my father wanted to identify the toxic substance in cigarette smoke and find a filter to produce smoke "suitable for the lungs." Now our vacations were moved to the French-speaking Switzerland, the laboratory of F.J. Burrus & Cie. In addition, he found a small room in the cellar of the schoolhouse to work before and after his lessons.
His first experiments were with thin-layer chromatography, but smoke produced only a brown band from the bottom to the top of the plate. The work with packed GC columns (and thermal conductivity detection [TCD] — flame ionization detection [FID] was not available yet) was a bit more successful: it provided some separation of the most volatile components of the gas phase, but he decided that this was not the adequate tool. At this time, petroleum scientists were employing stainless steel capillary columns, some more than 100 m in length and several kilograms in weight. In 1960, my father purchased such tubing, but found that the separation achieved on the more polar compounds in cigarette smoke was still difficult. Using polar stationary phases, such as tetraethylene glycol dimethyl ether (TED) or dinonyl phthalate, and programming the oven temperature from 0 to 60 °C, he could identify 38 components, ranging from acetone to toluene (Figure 2) (1). Identification involved the addition of standards to the smoke followed by the preparation of a synthetic mixture of about the same composition, and the quantitative comparison of this mixture with the cigarette smoke on two or more columns coated with different stationary phases — though without integrators. As a boy, I was impressed by the huge apparatus that stood on the floor and towered over me. It was connected to a recorder that was some 80 cm in each dimension, with two heavy motors, one to move the pen up and down over the paper that was driven by the other. The up and down of the peaks was accompanied by a singing sound — at modest speeds, but in these early days, this was not the most serious problem.
Figure 2
In 1961, he heard about the glass capillary columns of H.D. Desty and his drawing machine enabling him to pull capillary tubing in almost unlimited quantities (2). He asked Hansjürg Jäggi, the technician at his school, to build such a machine. The preparation of inert columns from this tubing was again difficult: most peaks were distorted, and it took several years to find ways to modify the glass surface so that it was sufficiently inert (suppressing adsorption of polar components) and at the same time wettable by the stationary phase (so that a homogeneous film could be spread on the surface). The coatings of polar stationary phases providing good deactivation tended to contract and form droplets.
The first success came with a surface modification that involved the pyrolysis of methylene chloride inside the column. It was never fully clarified whether it was the hydrochloric acid or the carbon that deactivated and furnished a roughened surface suitable for coating with medium polarity stationary phases, such as polyglycols (Carbowaxes) or polyglycol ethers (for example, Ucon, Emulphor), which were the most successful at that time.
In 1966, my father published chromatograms of cigarette smoke with hundreds of peaks between the gaseous components (such as carbon monoxide) and acetyl furan or isopropyl toluene. Now, identifications involved a large magnetic sector mass spectrometer that consumed an enormous amount of electricity. Scans were initiated manually and recorded on UV-sensitive paper, the masses were not displayed (but had to be counted up), and there was no library against which spectra could be compared. He identified 168 components (maximum column temperature, 100 °C) (3). In 1969, he added 133 components of lower volatility (from dimethyl pyridine to phenanthrene) in a chromatogram showing many hundreds of other peaks and with a resolution not surpassed by more modern GC (Figure 3). Using Emulphor (polyethylene glycol octadecyl ether) as a stationary phase, the temperature program reached 180 °C (4).
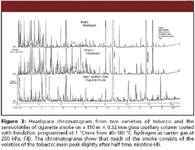
Figure 3
Some chromatograms lasted more than 2 h (for example, Figure 3), and when the attenuation was not well adjusted or the ink of the recorder dried, the analysis had to be repeated. Chromatograms often exceeded 1 m in length.
Some of these chromatograms showed almost 1000 peaks, but at the same time, the hope that cigarette smoke could be filtered to render it "suitable for the lung" came to an end. Despite toxicology being far from that available today, it was obvious that smoke would contain hundreds of toxic compounds and an effective filtration would never be sufficiently selective to allow more than some warm air to pass.
GC as the Discipline on Its Own
My father was not really disappointed about the failure to produce healthy smoke (he never smoked) and in the meantime, his main interest had become GC as a technology. The main efforts went into the preparation of better glass capillary columns, which meant chemical modification of the glass surface. He also tried synthesizing stationary phases grafted to the glass or making a plastic tube inside the glass tube, such as polybutadiene or perfluorated polymers, onto which he deposited the stationary phase (5).
The few users of capillary columns of that time usually made their columns themselves by recipes that were not very openly dealt with. The first commercial (glass) capillary columns were produced starting in 1967 by Jäggi, the technician of my father's school. He produced them in the living room of his old (wooden!) farmhouse, primarily for the flavor and fragrance industry, where the introduction of high-resolution GC enabled spectacular progress.
In 1968, my father invented splitless injection: one morning he forgot to open the split valve before injection and to his surprise the peaks were not only much larger, but also perfectly sharp — which everybody at that time thought to be impossible (6). Because he did all the work by himself, he noticed that this was more than a simple error and investigated the finding. It was the subject of the first of his lectures I attended, of which I understood primarily the starting point: how could a substance leave the column as a peak of at most a few seconds width if it entered the column during a splitless period lasting, for example, a minute? Indeed, splitless injection relies on the reconcentration of the sample in the column inlet, and it was one of his achievements of the following years to describe cold trapping and the solvent effects, the two mechanisms that can be used to shorten chromatographic bands (for example, see reference 7, which describes the first work he had me participating). Others were the optimization of the injector geometry and the introduction of the septum purge. This came right at the starting time of trace analysis, in which he also played a leading role (see below).
It was around 1970 when he also optimized capillary GC in various other aspects, such as the suspension of the glass capillary column in the oven, the straightening and treatment of the column end sections (Figure 4), the safe use of hydrogen as carrier gas, the coupling of columns to the GC–mass spectrometry (MS) interface with shrinking PTFE tubing, and various instrumental adjustments. In this way, glass capillary GC became a fairly mature technique that could be recommended for routine application. In Europe, the number of users grew substantially, but in the U.S. it remained an exotic technique for many more years.

Figure 4
In his eyes, there remained a serious gap: a simple and reliable method for column preparation that would render it easy for every laboratory to make its own columns. After great efforts in several directions, in 1976, he published the barium carbonate procedure (8) from which he expected a breakthrough. Onto a glass capillary surface leached with hydrochloric acid to form silica, barium carbonate was precipitated from a barium hydroxide solution, which was pushed through the capillary with carbon dioxide. A carefully selected detergent kept the aqueous film stable for a moment and a plug of air prevented precipitation from the bulk of the solution — an ingeniously designed procedure. The typically rather polar stationary phases were deposited after deactivation by "burning in" polyglycols to the surface.
However, the barium carbonate procedure was not the great success that he envisioned, also because he himself found an even better method. In the late 1970s, he learned from Thomas Welsch (9) that proper silylation of silica required a treatment at conditions as drastic as 300 °C for 20 h: more superficial derivatization disappears in the bulk of the glass upon modest heating, which explained all his (and others') previous failures. Now, he combined his acidic leaching of the glass surface to produce a layer of rather pure silica gel with a carefully optimized dehydration, leaving behind a concentration of surface silanols resulting in the best coverage with silyl groups, and introduced the diphenyl tetramethyl disilazane to improve wettability. This resulted in columns of high inertness even with low polarity polysiloxane stationary phases, which now became the most frequently used phases. Due to the chemical inertness of the support surface, the temperature limit went up from some 240 °C to at least 370 °C (10). In the following years, he continued refining this process, which in principle remains the most important path of column preparation to this day.
In 1979, fused-silica capillary tubing was introduced, which circumvented the need to straighten the end sections (glass capillaries were coiled after drawing). No doubt, fused silica is less fragile than glass, but this was more of a psychological than a real advantage. Now, capillary GC rapidly became popular and at the same time, the commercial columns started to dominate the homemade ones. Both developments questioned the continuation of the work of my father. He went on teaching courses on column preparation, but his conviction that every larger laboratory should master the technology of column preparation did not materialize and this know-how rapidly disappeared — our laboratory might well be almost the only one left preparing its own columns (although with fused-silica tubing).
Development of column preparation required quality evaluation. In 1968, my mother, Gertrud, began working mornings with my father, mainly testing the three to five columns he produced per day. She had a cabinet filled with small bottles of the various test mixtures, each for a given quality aspect and adjusted to some stationary phases. She knew perfectly well which mixture to run at what temperature (isothermal) for a given column. A better column test procedure was the first subject for the young son (me), confident that he could do better. It resulted in a test that became the standard for many years (1978). I called it "grob-Test" (German for "coarse test"), but my father turned it into "Grob-test."
In the early 1970s, my father bought the first integrator for about $20,000 (Infotronix). It printed out retention time and peak areas on a paper strip like the old cash registers in the food shops. He did not use it often, but frequently enough to notice that GC was a poor technique for quantitative analysis — and this was exactly what became the ever-more important application of GC. It was clear to him that the injection was the principal weakness. First, he improved the design of the split–splitless injector another time into what it essentially is today (11), but returning from a meeting where he heard Gerhard Schomburg describing his "inverted cup" on-column injection, he was convinced that on-column injection was the solution. He spent a morning on the phone and digging in catalogs to find steel tubing that was thin enough to serve as syringe needles entering the capillary columns (at that time usually being of 0.28–0.35 mm i.d.). In 1979, Carlo Erba Strumentazione (now Thermo Fisher Scientific, Milan Italy) brought an instrument on the market that incorporated an on-column injector (12).
Environmental Analysis
As the analysis of cigarette smoke became pointless, my father started analyzing organic compounds in ambient air and water. Around 1970, he developed a filter holder containing two minute traps, the second to detect breakthrough through the first. To maximize sensitivity he selected a trapping material of high capacity, charcoal, such that he could sample several cubic meters of air on 25 mg of adsorbent (between glass fiber disks) — making use of his experience in filtering cigarette smoke. He also constructed a small glass apparatus to extract the traps with some 0.4 mL of solvent, a bit Soxhlet-like (13). Then, his students collected air samples indoor and outdoor in the town of Zurich (Figure 5) as well as in remote forests. It was surprising to see that even in a kitchen with an obtrusive smell of food, little more than gasoline could be detected. Also finding pinene in a remote forest with a fresh smell of "clean" air was difficult. He looked at daily changes and meteorological effects — many years before others started doing such analysis. He published the first results in the newspaper of Zürich (14) — I do not believe he even thought about publishing in more prestigious journals like Nature or Science.
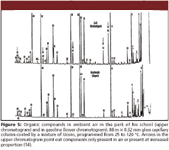
Figure 5
In 1972, he developed a method for analyzing trace organics in surface and drinking water: the closed-loop stripping procedure (15). Again, high sensitivity was achieved by miniaturizing the key items. Air was circulated through 1–5 L of water, a small charcoal trap, and the water again. The trap consisted of 1–2 mg of charcoal between small sieves. Breakthrough of the most volatile components did not matter, because they were re-extracted by the circulating air. Analytes were eluted from the trap with 5–8 μL of carbon disulfide by using small thermal changes to move the solvent up and down through the trap and to end up in a minute vial. This enabled the injection of the extract of a whole liter of water. Again, the chromatogram was crowded by gasoline peaks, but there were also other components (such as tri- and tetrachloroethylene).
I remember well a course he gave in Oslo, Norway in 1974: I was his assistant and analyzed the local tap water. The chromatogram was in the newspaper of the next day. The Norwegians were deeply disappointed about the many organic substances in their "clean" water — perhaps not fully aware of the fact that small peaks represented hardly 1 ppt (ng/L), despite using simple FID — it was the beginning of trace analysis.
This milestone in environmental analysis prompted him to move into a laboratory of the Swiss Federal Institute of Aquatic Science and Technology (EAWAG), where he was "permanent guest scientist" for most of the time up to his retirement.
My father retired in 1985. He phased out his work by long trips with my mother, combining practical teaching of GC with visiting other countries and cultures. In 1987, he had hardly started to seriously consider settling down when he suddenly died after jogging on an early morning. It seems his profession was his elixir and without this, the flame went out. He left behind my mother, who had always hoped to live a more quiet time with him after his retirement. She worked in GC to be with my father, less because of interest in his endeavors. She died in 2006.
His Way of Working
Considering how much my father achieved virtually alone and in his spare time — all while being a teacher — he was incredibly productive. In several sectors at the same time, he progressed faster than large research groups with far more equipment and resources: he developed much of capillary GC (column technology and injection techniques), was far ahead of everyone else in the analysis of cigarette smoke, pioneered in environmental analysis, but also did, for instance, the analysis for determining the substance ants use for tracing the path to feed sources. He published about 100 scientific papers, usually written in the vacation house in the mountains. No doubt, he was a highly gifted and intelligent researcher, but the working philosophy also played a role, and this is the aspect worth being investigated.
My father shaped his professional carrier outside the normal paths. He wanted to run on his own and did nearly everything to avoid being captured by a professional structure. In the classroom, he was his own boss. His style of teaching must have been far removed from normal, shaped by his character and beyond pedagogical scheme, but because he was successful, no one objected.
GC was his hobby and he loved it as perhaps only hobbies can be loved. He started work at 5 a.m., then went to school at 7 or 8 a.m. He ate lunch in the laboratory and came home at about 6 p.m. Schools were still open on Saturday morning during most of his professional life, but he did not go to the laboratory on Saturday afternoon or Sunday. During evenings and weekends he read some general scientific literature, but for the rest of the time he relaxed, for example, taking long walks with me. Occasionally he seemed a bit absent when we discussed daily matters at the table, but in general he was really (even mentally) at home.
He was not really paid for the GC work he did at Zürich. The cigarette producer F.J. Burrus & Cie. covered his lab expenses (initially hoping for healthy cigarettes) and when he worked at the EAWAG, this institute paid for the solvents, the gas, and the phone. Payment for a good part of his work on cigarette smoke was that one day our family received the money to build a vacation house in the mountains, including covering the related taxes. The university cared about the course he taught in GC by payment to the school to relieve him of some lessons. Financially this was not rewarding (some would probably say it was stupid), but he earned the money he needed and he would not have accepted any compromise to get more. The certainty that no one was entitled to ask something from him was part of his feeling of being free.
During most of his activity, Gertrud, his wife, was his help, working some 4 h in the mornings (paid by F.J. Burrus & Cie.; Figure 6). He never accepted another collaborator or student: he wanted to do it all by himself. He prepared all the several thousand columns, spent days in constructing his traps and other devices, as well as the tools to make these, such as minute burners or tungsten points to widen a capillary entrance. Usually such time was well invested, as it opened the door to new science. Manual work inspired his ideas and doing things all by himself ensured that he made all the relevant observations. He believed that having people working for him would not have accelerated progress, but just poured sand into his machine.

Figure 6
This description might make you think he was an unsocial person or even an anarchist. No doubt, he did not spend time singing and drinking beer in pubs, but he was neither shy nor did he hate people or was a loner. He voted for conservative parties, cared about wearing a tie for his students (that in Figure 1 was no exception), and had quite a career in the military. His refusal to do research in a social network resulted from his need to be free in what he was doing, but also from his conviction that he would be far more efficient in this way – which he demonstrated over and over again. He never wrote project proposals, had little administrative work to do, no "quality assurance," and no meetings (during which he would have fallen asleep).
Humans live by an internal fire and it would have been interesting to understand what fueled his fire. He only seldom participated at congresses, to some extent because of difficulties to arrange it with the school schedule, but primarily because he did not feel comfortable there. He knew that he did outstanding work and, perhaps, did not want to be confronted with what others made of this. He seemed totally uninterested in honors; it was enough that he and my mother were pleased by what they achieved —he would not need to hear it from others (probably a Protestant attitude). For him, success was not a tool to get into a higher social class (such as being professor or becoming rich), but satisfaction by its own. Nevertheless, as he received a second doctor honoris causa, this time from the Swiss Institute of Technology (ETH) in Zurich, he hung the diploma up on the wall at home.
My father was a scientist of the "old school," driven by curiosity and admiration of nature and technology. He was fascinated by all sorts of scientific discoveries and loved to teach, but he also loved to participate in the discovery process himself. Commercial applicability was not his interest — not really even if he had achieved a "healthy" cigarette. He was fascinated by the research process, the getting onwards step by step, creating ideas and clever devices, but also by lighting fires in the eyes of his students. He lived by the enjoyment of the moment.
This independence from institutions and external recognition eliminated stress from outside and allowed him to focus his mind and energy on work. He worked fairly long hours, and these intensively, but he was not a workaholic. I do not remember ever having seen him stressed. If something did not work, he had the time to investigate the matter and learn from it. It is well known that these are ideal conditions to be creative and productive.
In conclusion, I believe that the success of my father was the combination of an extraordinary intelligence and manual capability combined with keeping his "system" extremely simple: doing it all virtually on his own enabled him to focus all his attention to what he wanted to do. This was possible because he did not care about hierarchy, earnings, and prestige.
References
(1) K. Grob, Beiträge zur Tabakforschung, 285–290 and 315–323 (1962).
(2) D. H. Desty, J. N. Haresnape, and B. H. Whyman, Anal. Chem. 32, 302–304 (1960).
(3) K. Grob, Beiträge zur Tabakforschung 3, 403–408 (1966).
(4) K. Grob and J.A. Völlmin, Beiträge zur Tabakforschung 5, 52–57 (1969).
(5) K. Grob, Helvetica Chim. Acta 51, 718–737 (1968).
(6) K. Grob and G. Grob, J. Chromatogr. Sci. 7, 584–591 (1969).
(7) K. Grob and K. Grob Jr., J. Chromatogr. 94, 53–64 (1974).
(8) K. Grob and G. Grob, J. Chromatogr. 125, 471–485 (1976).
(9) T. Welsch, W. Engewald, and C. Klaucke, Chromatographia 10, 22–28 (1973).
(10) K. Grob, G. Grob, and K. Grob Jr., J. High Resol. Chromatogr. & Chromatogr. Commun. 2, 31–35 (1979).
(11) K. Grob and K. Grob Jr., J. High Resol. Chromatogr. & Chromatogr. Commun. 1, 57–64 (1978).
(12) K. Grob and K. Grob Jr., J. Chromatogr. 151, 311–320 (1978).
(13) K. Grob and G. Grob, J. Chromatogr. 62, 1–13 (1971).
(14) K. Grob and G. Grob, Die Verunreinigung der Zürcher Luft durch organische Stoffe, insbesondere Autobenzin. Neue Zürcher Zeitung August 7 (1972).
(15) K. Grob, J. Chromatogr. 84, 255–273 (1973).

Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.












