Shape Selectivity in Reversed-Phase Liquid Chromatography
LCGC North America
A variety of chromatographic sorbents are commercially available for reversed-phase liquid chromatography (RPLC) and while many of these columns are nominally similar, in practice the columns may provide significantly different separations.
A variety of chromatographic sorbents are available commercially for reversed-phase liquid chromatography (LC), and while many of these columns are nominally similar, in practice, the columns can provide significantly different separations. An understanding of the nature of these differences is essential for efficient and appropriate method development. Column differences are evident particularly for the separation of geometric isomers. Isomer separations often represent a considerable challenge because the compounds have similar chemical and physical properties but differ in molecular shape. The ability to separate and quantify the individual isomers within a specific class of compounds has significance because biological processes are highly dependent upon molecular shape. For example, the toxicity and carcinogenicity of polycyclic aromatic hydrocarbons (PAHs) and the bioavailability of fat-soluble vitamin isomers is influenced by their molecular shape. Thus, bioactivity can vary dramatically among isomers. Certain chromatographic sorbents provide enhanced separations of shape-constrained isomers; these phases are described as "shape selective." Extensive research has examined the role of stationary phase ligand density, chain length, and separation temperature on shape-selective separations. Through chromatographic tests, spectroscopic investigations, and molecular simulations, a clear picture of the factors that give rise to shape-selective phases has emerged. This article provides an overview of the investigations performed primarily at the National Institute of Standards and Technology (NIST) on alkylsilane chromatographic sorbents; for more detailed information regarding this work, please consult recent comprehensive literature reviews (1,2).

Ronald E. Majors
Chromatographic Evaluation of Shape Selectivity
Chromatographic test mixtures: Based upon observations of chromatographic retention and molecular shape, Wise and colleagues (3) developed a molecular descriptor of solute shape from the ratio of the length-to-breadth of a box drawn around the solute molecule oriented to yield the maximum value (L/B ratio). Long narrow solutes (that is, those with large L/B ratios) were retained longer than more compact solutes with smaller L/B values, particularly for shape-selective columns. The evaluation of L/B ratios and PAH retention data led to the development of Standard Reference Material (SRM) 869 Column Selectivity Test Mixture for Liquid Chromatography (4) (currently available as SRM 869b). SRM 869 is a mixture of three PAHs in acetonitrile: dibenzo[g,p]chrysene (alternate name, tetrabenzonaphthalene, TBN, MW = 328, L/B = 1.095) benzo[a]pyrene (BaP, MW = 252, L/B = 1.493) and phenanthro[3,4-c]phenanthrene (PhPh, MW = 328, L/B = 1.074). Molecular representations of the solutes of SRM 869 and its separation on shape-selective and nonshape-selective phases are shown in Figure 1. A selectivity factor of αTBN/BaP > 1.7 is indicative of a stationary phase with low shape selectivity. Values of αTBN/BaP between 1.0 and 1.7 are considered "intermediate," and values below 1.0 are indicative of a stationary phase with increased molecular shape-recognition characteristics. Shape-selective phases retain planar solutes longer than nonplanar solutes. SRM 869 can be used to predict the ability of a chromatographic sorbent to separate complex PAH mixtures as well as other mixtures of shape-constrained isomers, such as carotenoids and polychlorinated biphenyls (PCBs).
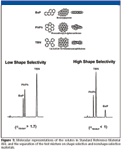
Figure 1
Other approaches used for the evaluation of the molecular recognition characteristics of chromatographic sorbents include the Tanaka test (5) and the separation of carotenoid isomers (6). The Tanaka test utilizes the ratio of the retention of triphenylene (TRI; planar shape) to o-terphenyl (o-TER; highly twisted shape). Values of αTRI/o-TER > 3 represent phases with enhanced shape selectivity, whereas values αTRI/o-TER < 2 suggest a phase that has low molecular-recognition characteristics.
Chromatographic sorbents possessing longer alkyl ligands, such as C30 and immobilized polymers, usually are shown to be shape selective when tested with SRM 869; however, other differences exist among the stationary phases. The separation of β-carotene isomers allows for an improved distinction among these chromatographic sorbents. Polymeric C30 phases offer increased selectivity of the β-carotene isomers when compared with monomeric C30 phases. Consistent with PAH isomer separations on C18 phases, the polymeric C30 phases typically show greater retention of the more linear trans-β-carotene and 9-cis-β-carotene isomers than the 13-cis and 15-cis isomers, which are bent near the midpoint of the molecule (7,8).
Effect of bonding density: The majority of stationary phases for reversed-phase LC are synthesized through the covalent modification of silica with alkyl silanes. Monomeric phases usually are prepared through the reaction of dimethylchloroalkyl silanes with the silica surface. This synthetic route results in a single siloxane bond from the reaction of the silane with the oxygen of a silanol on the silica surface. The concentration of reactive silanols on the silica surface is accepted as 8 μmol/m2 (9). In practice, the surface coverage of monomeric phases is limited to 4 μmol/m2 coverage and the immobilized ligands are distributed relatively evenly across the surface due to steric hindrance from the dimethyl groups of the silane reagent near the surface. Solution polymerized phases are prepared with trifunctional silanes in the presence of a precisely delivered aliquot of water. It is generally accepted that silane polymerization initially occurs in solution, followed by bonding of the polymers to the silica surface. 29 Si nuclear magnetic resonance (NMR) studies indicate that the polymers are, on average, five-unit oligomers, and are linked to the surface at three or four points (10). The solution polymerization approach allows for phases with higher surface coverage (4–5.5 μmol/m2 ), resulting in a sorbent with high-density regions of ordered alkyl ligands. An alternate polymeric modification approach reacts trichlorosilanes in the presence of hydrated silica (11). The polymerization occurs via self-assembly at the surface and higher density stationary phases up to 8 μmol/m2 coverage can be prepared. The near-complete surface coverage for these phases generally results in poor mass transfer kinetics in the separation process if a single length alkyl silane is used for the sorbent preparation. Alternatively, surface polymerized phases can be synthesized with alkyl ligands of mixed lengths; these phases exhibit shape recognition representative of monomeric-type phases (11–13).
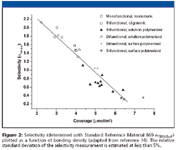
Figure 2
Ligand density plays an important role in shape recognition. In nearly every study of chromatographic shape selectivity, phases with higher ligand densities, greater than about 4 μmol/m2 , have been observed to exhibit better shape selectivity than lower density phases. This has been attributed to differences in the bonding chemistry because higher density phases are prepared exclusively by polymeric surface modification chemistry. Figure 2 shows a plot of alkyl ligand density versus αTBN/BaP for a range of C18 phases that have been prepared and characterized at NIST over two decades (14). Even though differences due to bonding chemistry and base silica exist among the phases, it is evident that there is a strong correlation between the ligand density and shape selectivity.
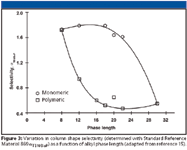
Figure 3
Effect of ligand length: The influence of alkyl ligand length on shape recognition is the subject of numerous studies. Figure 3 shows the effect of chain length on shape selectivity (as measured with SRM 869) for monomeric and solution polymerized phases (15). Both monomeric and polymeric C8 phases exhibited reduced ability to differentiate molecular shape (αTBN/BaP ≈1.7), however, both phase types had comparable values of αTBN/BaP (≈0.5) at an alkyl ligand length of C30. Additionally, the monomeric phases show little change in shape selectivity among the C8, C12, C16, and C18 lengths with a significant increase in selectivity between the chain lengths of C18 and C30.
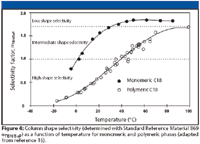
Figure 4
Conversely, the polymeric phases with chain lengths from C16 to C30 exhibit a significant degree of shape recognition that falls off rapidly below the length of C12.
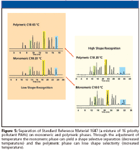
Figure 5
Effect of Temperature
For alkyl ligand stationary phases, an increase in shape selectivity generally is observed with a decrease in temperature. Figure 4 shows the dependence of PAH shape selectivity upon temperature for both monomeric and polymeric C18 stationary phases (16). For both types of chromatographic sorbents, a decreased value of αTBN/BaP is observed with reduced temperature. Separations of SRM 1647 Priority Pollutant Polycyclic Aromatic Hydrocarbons (in Acetonitrile) on monomeric and polymeric C18 stationary phases are shown in Figure 5. At low temperature, the separation with the monomeric C18 phase is very similar to the separation with the polymeric C18 phase at ambient temperature. This illustrates the important influence that temperature has on the shape-selective nature of a stationary phase and how it can be used to tune and optimize a separation.
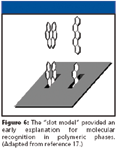
Figure 6
Spectroscopic Studies
From previous chromatographic studies that demonstrate an increase in shape recognition with increasing alkyl ligand density, chain length, and decreasing temperature, it was hypothesized that "slots" were formed as the ligands on the surface became more ordered. The slot model, shown in Figure 6, was presented in 1985 as a hypothesis for the genesis of shape selectivity within a phase that contains a high degree of molecular order (based upon the three primary factors of density, length, and temperature) (17). Together with empirical chromatographic evidence, the presence of such molecular features has been supported by numerous spectroscopic studies. Table I summarizes the most commonly used spectroscopic techniques for the evaluation of chromatographic sorbents with information on the phase characterization results offered by each technique (1). Infrared spectroscopy, Raman spectroscopy, and nuclear magnetic resonance will be discussed briefly.
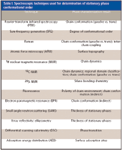
Table I: Spectroscopic techniques used for determination of stationary phase conformational order
Infrared Spectroscopy
Sander and colleagues (18) were the first to utilize infrared spectroscopy to study monomeric stationary phases that ranged in length from C1 to C22 at a range of temperatures from –30 °C to 47 °C. The signal intensity at 1367 cm–1 kink(gtg')/gtg and 1354 cm–1 bend was found to decrease with decreasing temperature while the signal at 1345 cm–1 (end gauche) was seen to increase. The spectra shown in Figure 7 indicate that a greater number of alkyl ligands are in the all-trans configuration at low temperature and, thus, represent an increase in ordering of the stationary phase.
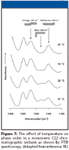
Figure 7
The CD2 rocking vibrational bands at 600 cm–1 to 680 cm–1 were studied in selectively deuterated phases to assign trans–trans and trans-gauche conformations to the labeled section of the alkyl ligands (19). Increased density, increased length, and decreased temperature were correlated with increased alkyl chain order. In addition, this research indicated that there was an increase in alkyl chain disorder closer to the silica surface. The results are consistent with a more recent investigation of the effect of surface coverage on the conformation and mobility of C18 ligands covalently bonded to silica (20).
In another investigation, the CH3 symmetric stretch peak at 2872 cm–1 and the CH2 symmetric stretch peak at 2840 cm–1 for surface polymerized phases were studied (21); this ratio of ICH3/ICH2 is indicative of the overall order of the alkyl chains on the surface. This research confirmed previous Fourier-transform (FT)-IR studies of alkyl-modified silica materials, and it showed that the confor mational order of mixed C18/C1 monolayers does not change significantly in the presence of solvent. Finally, these studies indicate that there is no significant evidence of "phase collapse" within alkyl-modified chromatographic surfaces.
Raman spectroscopy: Raman spectroscopy has been used to study the influence of solvent composition, temperature, and pressure on stationary phase conformational order. Raman spectroscopy does not suffer from interferences from water; however, there can be significant interferences from fluorescent species, such as impurities in the underlying silica. The intensity ratio of the antisymmetric to symmetric methylene bonds (I[νs (CH3)]/I[νs (CH2)]) is measured in Raman spectroscopy and correlated with the degree of alkyl ligand order. The nature of solvent environment was observed to have little influence on ligand order (22–24). Consistent with the FT-IR studies, an increase in ordering was observed with a decrease in temperature (25,26). The effect of surface coverage was investigated by Ducey and colleagues (22,25), for which an increase in density led to an increase in ligand order. Finally, the work performed by Dorsey and colleagues (27–) indicates that there is not a sharp phase transition over a wide range of aqueous–organic mobile phase compositions and suggests that a phase collapse process does not occur.
NMR
NMR has offered significant insights into the molecular ordering of chromatographic surfaces and is the subject of an in depth review by Albert (30). As indicated in Table I, different nuclei can be used to gain information about the underlying chemical structure of the chromatographic sorbent.
29Si cross-polarization magic angle spinning NMR provides information about the nature of the bonding of the alkyl ligands to the silica surface as well as details regarding the polysiloxane backbone in polymeric phases. In one recent study, the chemical bonding differences between a solution polymerized and surfaced polymerized C18 stationary phases was investigated. It was found that although the overall densities of the phases were different, a comparable degree of polymerization was observed (31). Another study demonstrated that in highly crosslinked surface polymerized phases, only about 30% of the silicon atoms are bonded to the silica support, which allows for some flexibility in the polysiloxane backbone (32). This flexibility can result in increased ordering of alkyl ligands on the surface.
A number of 1 H and 13 C NMR techniques have been utilized to study the molecular ordering of the bonded phase where the shift in signal from 30.0 ppm to 32.6 ppm indicates the increased ordering of the phase. Figure 8 summarizes a series of experiments in which NMR was used to investigate the effects of alkyl ligand density, ligand length, and temperature on alkyl ligand order (33). The effects of the three parameters are consistent with other chromatographic and spectroscopic data.
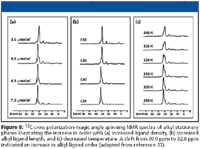
Figure 8
Spin diffusion solid-state NMR has been used to probe the size of the regions of order and disorder within the bonded phase (34). It was determined that C30 chains form distinct regions of order and disorder, in which the ordered regions have an average size of 82 nm2 and the disordered areas were 125 nm2 . In addition, the overall length of the chains was measured as 4.3 nm and 3.1 nm in the ordered and disordered regions, respectively.
Molecular Simulations
Molecular modeling techniques that are based upon statistical mechanics (Monte Carlo [MC], molecular dynamics) can be used to provide insight into the molecular-level structure and function of a bonded phase, given that direct experimental observation currently is not feasible. By and large, MC simulations rely on statistical sampling of a molecular system and are arguably the preferred method for the determination of the averaged thermodynamic properties, whereas molecular dynamics (MD) describes the motion of all particles within a system simultaneously and can better depict the growth of molecular-level features of a system over a specified time period. MC and MD simulations are regarded as dissimilar yet complementary techniques and there has been considerable effort to combine the two into a variety of hybrid MD–MC methods (35).
MD simulations have been employed recently to depict the molecular-level organization of a range of nonsolvated monomeric and polymeric alkyl ligands on a larger-scale silica surface (4.1 nm × 5.9 nm) (36,37). Consistent with spectroscopic results, an increase in order of the alkyl ligands was observed with increased chain density, increased chain length, and decreased temperature. The simulation snapshots shown in Figure 9 demonstrate the effect of density on the molecular ordering of the stationary phase. These models represent an idealized chromatographic surface and, as with all molecular simulation results, can be interpreted only within their original design constraints. Comparable results, however, were observed for fully solvated molecular dynamics simulations for a series of C18 monomeric systems (38). As the chain density increases for the polymeric C18 models under nonsolvated conditions, the appearance of persistent, highly ordered cavities within the surface is noted (39). Evolution of such chromatographic surface features in these models is consistent with the originally proposed slot model and provides complementary information to the spectroscopic and chromatographic evidence of alkyl chain molecular ordering effects for these systems.

Figure 9
MC simulations of chromatographic models that describe the interactions of PAHs (up to four-member ring in size) within monomeric C18 phases have illustrated consistent agreement with experimentally determined retention time data (40). The presence of persistent and rigid slots within these models, however, was contested recently by Siepmann and colleagues (40) based upon their results for monomeric C18 systems with a maximum surface coverage of 4.2 μmol/m2 . In their model, PAH isomers exhibited specific positional and orientational distributions within the alkyl chains with alignment of their longest axis perpendicular to the silica surface. These preferential molecular recognition sites were observed to diminish at lower surface coverages, and they were not characterized as conformationally "rigid" because the alkyl chains were observed to interact freely with the solute and solvent throughout the simulation. The origin of the molecular recognition sites that stem from alkyl-modified chromatographic materials, however, is not apparent from the MC simulations. The most relevant modeled system represents a phase of moderate surface coverage for which shape selectivity is only beginning to emerge. It is not truly reflective of shape-selective polymeric materials (>5 μmol/m2 ) that are requisite for the challenging separations of larger-sized, shape-constrained isomers, such as five- and six-membered ring PAHs, carotenoids, and steroids. Prospective MC simulations of larger-scale systems with higher surface coverages and larger molecular weight solute probes should be able to address these points.
Results obtained from both MC and MD simulations provide complementary views of specific molecular-recognition sites within the chromatographic models that represent shape-selective materials. It is generally agreed that modeled results must be tested and compared with experimental results for validation before using for predictive purposes. MC simulations have been evaluated by comparison with thermodynamic measurements (that is, chromatographic retention data) (41), whereas MD simulations that provide structural information over time have been evaluated with spectroscopic measurements (for example, NMR, IR, and Raman spectroscopy). The consistency of molecular features that evolve under similar conditions (for example, higher bonding densities) from such diametric modeling approaches provides a broader understanding of chromatographic shape-selective processes.
Future Directions
The origin of shape selectivity in traditional hydrocarbon stationary phases based upon molecular ordering effects of the alkyl ligands has been well characterized. However, various other geometric isomers, such as steroids and halogenated flame-retardant compounds, might benefit from separations on chromatographic sorbents that contain additional moieties that promote both enhanced solvation and molecular ordering; the use of amine- and ether-embedded polar phases for the separation of such hydrophilic isomers is currently under investigation. In addition, the synthesis and characterization of extended-length fluorinated phases that can offer both rigid structural features together with unique electronic properties for the separation of halogenated species and larger biomolecules is presently underway.
Ronald E. Majors "Column Watch" Editor Ronald E. Majors is business development manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com.
Catherine (Kate) Rimmer, Katrice Lippa, and Lane Sander are research chemists within the Chemical Science and Technology Laboratory at the National Institute of Standards and Technology (NIST). Lane developed the first polymeric C18 and C30 columns to address separation challenges posed by isomers. Kate has investigated fundamental chromatographic processes through the design and characterization of novel sorbents, including fluorinated stationary phases. Katrice has utilized modeling and cheminformatics to provide insight into the origin of shape selectivity for hydrocarbon and fluorocarbon systems. Their research efforts are directed towards an improved understanding of chromatographic processes to advance the "state-of –the-art" in chemical metrology.
References
(1) K.A. Lippa, C.A. Rimmer, and L.C. Sander, Advances in Chromatography, E. Grushka, N. Grinberg, Eds. (CRC Press, Taylor and Francis Group, Boca Raton, Florida, 2008), pp. 235–303.
(2) L.C. Sander, K.A. Lippa, and S.A. Wise, Anal. Bioanal. Chem. 382(3), 646–668 (2005).
(3) S.A. Wise, W.J. Bonnett, F.R. Guenther, and W.E. May, J. Chromatogr. Sci. 19(9), 457–465 (1981).
(4) L.C. Sander and S.A. Wise, Certificate of Analysis, Standard Reference Material 869a, Column Selectivity Test Mixture (NIST, Gaithersburg, Maryland, 1998).
(5) N. Tanaka, Y. Tokuda, K. Iwaguchi, and M. Araki, J. Chromatogr. 239 (APR), 761–772 (1982).
(6) E. Lesellier, A. Tchapla, and A.M. Krstulovic, J. Chromatogr. 645(1), 29–39 (1993).
(7) L.C. Sander, K.E. Sharpless, N.E. Craft, and S.A. Wise, Anal. Chem. 66(10), 1667–1674 (1994).
(8) L.C. Sander, K.E. Sharpless, and M. Pursch, J. Chromatogr., A 880(1–2), 189–202 (2000).
(9) J. Nawrocki, J. Chromatogr., A 779(1–2), 29–71 (1997).
(10) H.O. Fatunmbi, M.D. Bruch, and M.J. Wirth, Anal. Chem. 65(15), 2048–2054 (1993).
(11) M.J. Wirth and H.O. Fatunmbi, Anal. Chem. 64(22), 2783–2786 (1992).
(12) M.J. Wirth and H.O. Fatunmbi, Anal. Chem. 65(6), 822–826 (1993).
(13) M.J. Wirth, R.W.P. Fairbank, and H.O. Fatunmbi, Science 275(5296), 44–47 (1997).
(14) L.C. Sander, M. Pursch, and S.A. Wise, Anal. Chem. 71(21), 4821–4830 (1999).
(15) L.C. Sander and S.A. Wise, Anal. Chem. 59(18), 2309–2313 (1987).
(16) L.C. Sander and S.A. Wise, Anal. Chem. 61(15), 1749–1754 (1989).
(17) S.A. Wise and L.C. Sander, J. High Resolut. Chromatogr. 8(5), 248–255 (1985).
(18) L.C. Sander, J.B. Callis, and L.R. Field, Anal. Chem. 55, 1068–1075 (1983).
(19) S. Singh, J. Wegmann, K. Albert, and K. Muller, J. Phys. Chem. B 106(4), 878–888 (2002).
(20) G. Srinivasan, L.C. Sander, and K. Muller, Anal. Bioanal. Chem. 384(2), 514–524 (2006).
(21) M.C. Henry, L.K. Wolf, and M.C. Messmer, J. Phys. Chem. B 107(12), 2765–2770 (2003).
(22) M.W. Ducey, C.J. Orendorff, J.E. Pemberton, and L.C. Sander, Anal. Chem. 74(21), 5585–5592 (2002).
(23) C.J. Orendorff, M.W. Ducey, and J.E. Pemberton, J. Phys. Chem. A 106(30), 6991–6998 (2002).
(24) J.E. Pemberton, M.K. Ho, C.J. Orendorff, and M.W. Ducey, J. Chromatogr., A 913(1–2), 243–252 (2001).
(25) M.W. Ducey, C.J. Orendorff, J.E. Pemberton, and L.C. Sander, Anal. Chem. 74(21), 5576–5584 (2002).
(26) M. Ho and J.E. Pemberton, Anal. Chem. 70, 4915–4920 (1998).
(27) C.A. Doyle, T.J. Vickers, C.K. Mann, and J.G. Dorsey, J. Chromatogr., A 779(1–2), 91–112 (1997).
(28) C.A. Doyle, T.J. Vickers, C.K. Mann, and J.G. Dorsey, J. Chromatogr.,A 877(1–2), 25–39 (2000).
(29) C.A. Doyle, T.J. Vickers, C.K. Mann, and J.G. Dorsey, J. Chromatogr., A 877(1–2), 41–59 (2000).
(30) K. Albert, J. Sep. Sci. 26(3–4), 215 –224 (2003).
(31) G. Srinivasan, C. Meyer, N. Welsch, K. Albert, and K. Muller, J. Chromatogr., A 1113(1–2), 45–54 (2006).
(32) M. Pursch, D.L. Vanderhart, L.C. Sander, X.H. Gu, T. Nguyen, S.A. Wise, and D.A. Gajewski, J. Am. Chem. Soc. 122(29), 6997–7011 (2000).
(34) M. Raitza, J. Wegmann, S. Bachmann, and K. Albert, Angewandte Chemie-International Edition 39(19), 3486 (2000).
(35) A.R. Leach, Molecular Modeling Principles and Applications; 2nd ed. (Prentice Hall, Upper Saddle River, New Jersey, 2001).
(36) K.A. Lippa, L.C. Sander, and R.D. Mountain, Anal. Chem. 77(24), 7852-7861 (2005).
(37) K.A. Lippa, L.C. Sander, and R.D. Mountain, Anal. Chem. 77(24), 7862-7871 (2005).
(38) A. Fouqueau, M. Meuwly, and R.J. Bemish, J. Phys. Chem. B 111(34), 10208-10216 (2007).
(39) K.A. Lippa and L.C. Sander, J. Chromatogr., A 1128(1-2), 79-89 (2006).
(40) J.I. Siepmann, J.L. Rafferty, and M.R. Schure, M. R. Understanding Shape Selectivity of PAHs in Reversed-Phase Liquid Chromatography via Monte Carlo Simulations, L-006 at the 32nd International Symposium on High Performance Liquid Phase Separations and Related Techniques, May 10-16, 2008, Baltimore, Maryland.
(41) J.L. Rafferty, L. Zhang, J.I. Siepmann, and M.R. Schure, Anal. Chem. 79(17), 6551-6558 (2007).

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












