Isolation and Recovery of Triclosan from Liquid Hand Soap Using Reversed-Phase Solid-Phase Disk Extraction and a Capillary GC-AED Determinative Technique
LCGC North America
Triclosan is an ubiquitous antibacterial, antimicrobial chemical found in numerous consumer health care products today. This article demonstrates that triclosan can be quantitatively determined in commercial hand soaps using reversed-phase solid-phase disk extraction coupled to quantification using capillary gas chromatography-atomic emission detection while avoiding emulsions.
Triclosan (5-chloro-2-[2,4-dichlorophenoxy] phenol) is a broad-spectrum antibacterial, antimicrobial agent classified as a Class III drug by the FDA. Triclosan has been used for over 30 years and is manufactured by Ciba Specialty Chemicals (Basel, Switzerland) in the U.S. and outside of the U.S. in countries such as Switzerland, the Netherlands, China, India, and South Korea. The FDA currently is assessing whether household use of products that contain triclosan lead to bacterial resistance as well as assessing risks to public health and to the environment (1). New research shows that triclosan has been found to hasten the transformation of tadpoles into adult frogs (2,3). More than 55% of streams examined in 2002 had a median triclosan concentration of 0.14 ppb (2,6). The molecular structure for triclosan is shown in Figure 1.

Table I lists products that contain triclosan (4). Poiger and colleagues at the Swiss Federal Research Station for Fruit-Growing, Horticulture, and Viticuluture, and the Institute of Environmental Chemistry at Umeå, University of Sweden, show that under certain conditions, triclosan can photodegrade rapidly, but methyl triclosan is relatively stable in the environment (5,6). Latch and coworkers at the University of Minnesota (Minneapolis and St. Paul, Minnesota) reported on the photochemical fate of triclosan. Triclosan rapidly photo-degrades with a halflife of 5 h in water at pH 8 under noon summer sunlight conditions to 2,8-dichlorodibenzo-p-dioxin and 2,4-dichlorophenol (7).
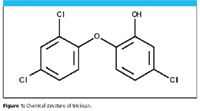
Figure 1
Current analytical approaches to the isolation and recovery of triclosan are focused on determining triclosan at trace concentration levels from matrices as diverse as human urine, surface water, marine sediments, and wastewater using solid-phase extraction (SPE) techniques and liquid chromatography–tandem mass spectrometry (LC–MS-MS) as the determinative technique (8–10). However, a literature search using the SCIRUS (Elsevier, Amsterdam, The Netherlands) search engine to find recent reference to methods developed for triclosan in liquid hand soaps is scant at best. Because I was engaged in developing an alternative sample preparation approach to isolating and recovering polybrominated diphenyl ethers (PBDEs) using reversed-phase SPE, I began to spike various matrices with triclosan. Triclosan is structurally similar to PBDEs. My studies on PBDEs were leading me to the use of reversed-phase solid-phase disk extraction (SPDE). I then wondered if I could extract the ubiquitous triclosan from commercial liquid hand soaps. I quickly discovered that, similar to other polychlorinated phenols, triclosan has sufficient hydrophobicity so as to be amenable to analysis by gas chromatography (GC).
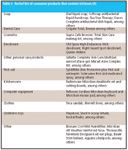
Table I: Partial list of consumer products that contain triclosan (4)
Capillary GC with atomic emission detection (GC–AED) complements other gas chromatographs in its ability to provide sufficient GC resolution with highly selective detection (11). GC–AED is a good choice when chemical constituents are to be quantitated from commercially formulated products at concentration levels from low parts-per-million to parts-per-hundred or percents. Triclosan is listed as the active biocide ingredient in most commercial liquid hand soaps most commonly at a concentration level of 0.15%.
This article demonstrates that triclosan can be isolated and recovered from commercial liquid hand soaps utilizing reversed-phase SPDE coupled to the capillary GC–AED determinative technique.
Experimental
Reagents, chemical reference standards, equipment: Triclosan was purchased from Calbiochem (San Diego, California). Dial antibacterial liquid handsoap (The Dial Corporation, Scottsdale, Arizona), Alberto VO5 (Alberto-Culver USA, Inc. Melrose Park, Illinois), and Meijer antibacterial liquid handsoap (Meijer, Grand Rapids, Michigan) were obtained locally. Focus, MP3SPEC, C2SPEC, C8SPEC, and C18SPEC disks were kindly provided (Varian, Inc., Lake Forest, California) in a 3-mL cartridge containing 20 mg of sorbent. A 24-port Visi-Prep vacuum manifold (Supelco, Inc. Bellefonte, Pennsylvania) was used to conduct all reversed-phase SPDE. A Vacuum/Pressure Station (Alltech, Deerfield, Illinois) provided the necessary vacuum. A 1-L safety-coated filtering flask with a side arm served as a water trap between the vacuum pump and the vacuum manifold. Empty SPE (60-mL) reservoirs also were used and connected to the 3-mL barrel cartridge using standard Luer adapters (Varian, Lake Forest, California). An N-EVAP Model 111 (Organomation, Northborough, Massachusetts) was used to apply a gentle nitrogen purge with a water bath heated to ~50 °C, Chemical reference standards containing triclosan dissolved in isopropanol were placed in 3-μL conical glass inserts with spring (MicroLiter Analytical Supplies, Inc., Suwanee, Georgia). The inserts were placed in 2-mL, 12 mm × 32 mm glass screw top vials (Target DP screw-thread vials, National Scientific, VWR, West Chester, Pennsylvania). The 2-mL vials containing the inserts were placed in the GC autosampler and automatically injected.
Instrumentation
A model 6890 GC system interfaced to a model G2350A atomic emission detector utilizing ChemStation software for instrument control and data processing (Agilent Technologies, Inc., Wilmington, Delaware). A 30 m × 0.25 mm DB-5 column (Agilent Techonologies, Inc.) was used to provide adequate retention of triclosan under temperature program conditions. The following temperature program was used to obtain all results reported in this article. For pulsed splitless injection, the initial oven temperature was 110 °C and held for 1.0 min and then increased to 225 °C at 120 °C/min and held for 0.5 min. The oven was then programmed to 300 °C at 10 °C/min and held for 2.0 min. A temperature programmed cool down also was included. For split injection, the first ramp was eliminated and the remainder of the temperature program was identical to the pulsed splitless approach. We observed a consistent retention time tR of between 4 and 6 min depending upon the oven temperature program used. The 837-nm atomic emission line for chlorine was monitored throughout the study, and the 834-nm atomic emission line for carbon was used for confirmation. A 1-μL volume of eluent from reversed-phase SPDE was injected automatically under both pulsed splitless and split injection techniques.
Sample preparation: Table II shows a step-by-step procedure that I developed for implementing the reversed-phase SPDE technique for isolating and recovering triclosan in various liquid hand soaps. This procedure for conducting reversed-phase SPDE with subsequent moisture removal evolved throughout the studies reported in this paper. The passing of various soaps, lotions, aloe gels, and shampoos through the conditioned reversed-phase SPDE cartridges occurred without any foaming. However, the effluent from each cartridge produced some foaming in the vacuum manifold itself and excessive foaming in the water trap flask. This foaming did not present any problems during reversed-phase SPDE.

Table II: Procedure to isolate and recover triclosan from a commercial liquid hand soap
Results and Discussion
Calibration and verification: Triclosan proved straightforward to analyze using GC on a 30 m × 0.25 mm DB-5 wall-coated open tubular column (WCOT). An initial stock chemical reference standard was prepared by dissolving 0.100 g triclosan in 10 mL isopropanol to yield a 10,000 ppm concentration of triclosan. Most liquid hand soaps list triclosan as the principal biocide at 0.15%. Based upon weighing 0.1-g soap while assuming a 100% recovery and a 200-μL final elution volume, ~750 ppm triclosan can be expected. A series of calibration standards were then prepared from this stock that covered the range from 50 ppm triclosan to 2000 ppm triclosan. This range of concentration levels is appropriate for a 1-μL injection into the GC–AED system provided we injected under split conditions while optimizing the split ratio at 25:1. Figure 2 shows GC–AED chromatograms that compare a triclosan control reference with a retention time of 4.98 min to an eluent from a triclosan-spiked aliquot of shampoo using reversed-phase SPDE techniques. Coefficients of determination for triclosan calibrations were consistently >0.9950 over this range of concentration levels. The AED emission line for chlorine at 837 nm was used for quantification. Initial calibration verification standards at 750 ppm triclosan (in isopropanol) were run routinely to verify the quality of the calibration over time.

Figure 2
Sample preparation: Initial attempts to use liquid–liquid extraction (LLE) by adding methylene chloride to liquid hand soap initially dissolved in distilled, deionized water (DDI) proved fruitless due to formation of intractable emulsions. However, these emulsions cleared over a three-week period. I was able to pass the upper phase (aqueous) through a conditioned Focus disk and recover some triclosan qualitatively.
Findings from unspiked commercial hand soap: Table III presents triclosan percent recoveries for three aliquots of a commercial hand soap (Brand A) and four aliquots of a second commercial hand soap (Brand B), implementing the procedure described in Table II. Results for duplicate injection of each eluent from Brand A assesses capillary GC–AED instrument precision. Results for triplicate reversed-phase SPDE for Brand A assesses extraction precision. Assuming that the 0.15% triclosan as stated on the label is accurate, three aliquots isolated and recovered via reversed-phase SPDE gave 46, 56, and 66% recoveries, respectively. Brand B are results for four aliquots of a hand soap that is not a name brand. The %triclosan recovery for Brand B based upon the same assumption is significantly lower.

Table III: Results for triclosan in liquid hand soap
An external standard mode of instrument calibration was used. The high selectivity afforded by quantitating only the 837-nm atomic emission line for chlorine eliminates some matrix effects. A postextraction spike can uncover additional matrix effects and should be considered when reproducing these experimental findings. A much larger number of sample aliquots from a given brand of any specific commercial hand soap would need to be extracted and analyzed to evaluate how close the experimental result is to the stated result on the label. Because I realized that I had succeeded in recovering triclosan to a certain degree, I proceeded to conduct a careful study of the percent recovery of triclosan. I spiked matrices of similar composition to liquid hand soap with triclosan that were not known to contain any triclosan and measured triclosan percent recoveries.
Effect of purge and chemical nature of disk on triclosan percent recoveries: I first considered an acidified DDI matrix. Lowering the pH of the sample should serve to suppress phenolic proton dissociation as well as to protonate surface silanol groups when using chemically bonded silicas.
~30 mL of acidified DDI with 100 μL of 5650 ppm triclosan (in isopropanol) and compared triclosan percent recoveries with and without the nitrogen blowdown and reconstitution step subsequently in this paper, referred to as the purge step. Triclosan percent recoveries varied from a high of 74% recovery to a low of 27% recovery. Using a Focus SPE disk, I spiked ~30 mL of acidified DDI with 100 μL of 5650 ppm triclosan (in isopropanol) to each of three reversed-phase SPDE cartridges. I eluted the sorbed triclosan with 1:1 methylene chloride–ethyl acetate. I did not evaporate to dryness. I adjusted to a final eluent volume of 1.0 mL using a volumetric flask. Results of this experiment are shown in Table IV. The mean percent recovery of trichlorosan % RTC was calculated according to the following equation:
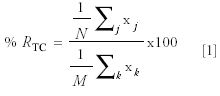
Where xj represents the recovered concentration of triclosan (in parts per million) for the j-th replicate injection for a given reversed-phase SPDE cartridge from the spiked matrix, N represents the number of replicate injections for a given solid-phase extraction, and xjC represents the concentration of triclosan (in parts per million) measured in k-th replicate injection, in a control. This control is a 100% recovered chemical reference standard dissolved in n-hexane. M represents the number of replicate injections of the control
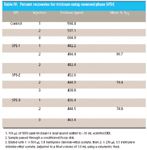
Table IV: Percent recoveries for triclosan using reversed-phase SPDE
This study established ~75% recovery of triclosan from spiked acidified DDI provided we did not include a purge step. It demonstrates the benefit of using the 1:1 methylene chloride–ethyl acetate as elution solvent for triclosan. After having achieved good triclosan percent recoveries via reversed-phase SPDE from spiked and acidified DDI, I proceeded to compare triclosan percent recoveries while including a purge step and varying the chemical nature of the disk itself. Results for this study are shown in Table V. Except for one relatively low triclosan percent recovery using MP3SPEC, this study demonstrated a >85% triclosan recovery and was quite consistent with respect to the other types of SPE disks used when a purge step was not included. Triplicate extractions using the Focus disk from spiked water including a purge step resulted in a much wider range of percent recoveries. I then decided to vary the sample matrix using other health care products that "looked" like liquid hand soap while keeping everything else the same.
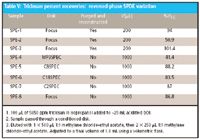
Table V: Triclosan percent recoveries: reversed-phase SPDE variation
Triclosan percent recoveries from various spiked products known not to contain triclosan: I took ~0.28-g amounts of a skin lotion, a gel containing aloe, and a shampoo. Triclosan percent recoveries varied from 30% to 51%. I also attempted to spike various lotion formulations with triclosan and found a wide variation in triclosan percent recoveries. I sensed that attempting to dissolve amounts of shampoo above 0.25 g made it very difficult to achieve reproducible triclosan percent recoveries. I then took a much smaller amount of shampoo, spiked it with triclosan and made repeated injections of the reversed-phase SPDE eluent. Results for this study are shown in Table VI. A 90% recovery of triclosan from spiked VO-5 shampoo was found. I began to look at the effect of soap or surfactant concentration in the aqueous sample on the percent recovery of triclosan with some interesting results. This study points to the need to conduct further triclosan percent recoveries from increasingly lower surfactant concentrations. At these low surfactant concentrations in water, the critical micelle concentration for a given surfactant might influence triclosan percent recoveries.
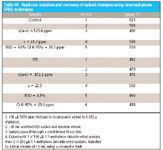
Table VI: Replicate isolation and recovery of spiked shampoo using reversed-phase SPDE techniques
Conclusion
The feasibility of using a reversed-phase SPDE technique to isolate and recover the environmentally significant biocide, triclosan, from commercial liquid hand soaps and related matrices has been demonstrated. Capillary GC–AED was found to be a most appropriate determinative technique for separating and detecting triclosan in eluents from reversed-phase SPDE at the concentration levels typically found in liquid hand soaps.
Acknowledgments
Larry Warren assisted in sample preparation and in researching triclosan use. Dee Childs (Varian, Inc, Consumable Products Division, Lake Forest, California) graciously supplied the various SPE disks used in this study. The Michigan Public Health Institute, the CDC Biomonitoring Planning Grant Program, and the Department of Health and Human Services, Center for Disease Control and Prevention, Public Health Emergency Preparedness provided support.
References
(1) B. Hilleman, Chem. Eng. News, October, 24, 14 (2005).
(2) J. Pelley, Environ. Sci. Technol. 41(1), 12–13 (2007).
(3) N. Veldhoen, R. Skirrow, H. Osachoff, H. Wigmore, D. Clapson, M. Gunderson, G. Van Aggelen, and C. Helbing, Aquatic Toxicol. 80(3), 217–227 (2006).
(4) A. Glaser, Pesticides and You 24(3), 12–17 (2004).
(5) R. Renner, Environ. Sci. Technol. 36(11), 228A–230A (2002).
(6) A. Lindstrom, I. Buerge, T. Poiger, P. Bergquist, M. Müller, and H. Buser, Environ. Sci. Technol. 36(11), 2322–2329 (2002).
(7) D. Latch, J. Packer, B. Stender, J. Van Overbeke, W. Arnold, and K. McNeill, Environ. Toxicol. Chem. 24(3), 517–525 (2005).
(8) X. Ye, Z. Kuklenyik, L. Needham, and A. Calafat, Anal. Chem. 77(16), 5407–5413 (2005).
(9) W. Hua, E. Bennett, and R. Letcher Environ. Int. 31(5), 621–630.
(10) A. Agüera, A. Fernandez-Alba, L. Piedra, and M. Gomez, Anal. Chimica Acta 480(2), 193–205 (2003).
(11) P.R. Loconto, Trace Environmental Quantitative Analysis, 2nd ed. (CRC Press, Taylor and Francis, Boca Raton, Florida, 2006), pp. 417–423.

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.











