Indentifying Packaging-Related Drug Product Impurities
LCGC North America
This month's installment of "MS - The Practical Art" provides a slightly different view of how practitioners employ the skills of interpretation that have been the focus in recent columns.
One recent column (1) examined a structured, methodical approach to characterizing suspected counterfeit active pharmaceutical ingredients (API). A logical conclusion this month provided by guest contributors from Cardinal Health (Research Triangle Park, North Carolina) takes the methodology a step further. Once a properly manufactured and tested drug has been packaged, there can be anomalies found on reanalysis after storage. The practitioners in these cases need to employ a variety of tools as well as their experience to come up with a confirmed identity for the anomaly as well as a possible source for its presence. Once again, mass spectrometers play a central role in the effort. Identifying drug-product-related impurities is a challenge that all pharmaceutical companies face. Compared with drug-substance-related impurities, which most commonly originate with the synthetic process or subsequent degradation, drug-product-related impurities can originate from several additional sources. Those sources include excipients and interaction with the packaging or container–closure devices. Although we can identify drug-product-related impurities solely via a sound analytical approach (2,3), considering and understanding all potential sources of those impurities can facilitate the process and expedite a solution.

Identifying impurities originating from the drug substance or excipients is not necessarily trivial, but at least those things lend a starting point for the investigation. But identifying impurities originating from the packaging or container–closure devices can sometimes prove more challenging for several reasons:
- The plastic and rubber materials used in the packaging and container–closure devices are often proprietary.
- The additives, potential leachables, are not always known.
- The manufacture of plastic and rubber materials does not usually proceed according to current good manufacturing (cGMP) conditions, and minor changes in process or technique can affect potential leachables significantly.
- Impurities often can appear as drug substance degradants that form on stability, which can lead the investigation astray.
- Some leachables can migrate through several layers of seemingly impermeable packaging, which can prove surprising, warranting the consideration of all components of the packaging or container–closure devices as potential sources of leachables.
Several researchers have published studies on impurities related specifically to the packaging of drug products (4–6).
After an initial assessment evaluating both ultraviolet and mass spectral data, if an impurity is categorized as unrelated to the drug substance or excipients, you should evaluate the packaging and container–closure devices. Review the literature as we have indicated in the references, or product information sheets (if available) from the manufacturer of the plastic or rubber materials used in the packaging or container–closure devices. Analyzing the components of the packaging or container–closure devices is also a viable option that can narrow the investigation, provide a source with a relatively greater amount of the impurity compared to the drug product, or provide a source not hindered by drug product excipients that can interfere with mass spectrometry (MS) analysis.
Here, we present several case studies illustrating how we identified leachables in drug-product formulations.
A typical scenario for these situations occurs during stability testing of a drug product when an unknown peak crosses the 0.1% threshold (or other defined threshold) at which impurities must be identified.
Example 1
Figure 1 shows a high performance liquid chromatography (HPLC)-UV chromatogram of an active pharmaceutical ingredient (API) stability assay for an injectable drug. In the chromatogram, an unknown peak is eluted at about 38 min, a peak we had not observed during the drug product analyses performed before the drug was stored in its final packaging (amber glass vial with gray, rubber stopper). In this relatively simple packaging scenario, the likely origin of any impurity that is container closure-related is the elastomeric stopper. But an assay solely by HPLC-UV makes it almost impossible to identify the impurity.

Figure 1
Even with diode-array spectra of the unknown versus the API (which requires minimal effort other than detector substitution), it is nonetheless difficult to discern the identification (Figure 2). Hence, an ideal scenario for a mass spectrometry solution to the problem.

Figure 2
The particular method involves no involatile buffers (for example, phosphate buffers) and, thus, requires minimal modifying to convert to an LC–MS method. When analyzed using negative-ion atmospheric pressure chemical ionization (APCI), a [M–H]- appears at m/z 339, suggesting a molecular weight of 340 Da. LC–MS-MS analysis showed a primary fragment appearing at m/z 163, an impurity that corresponds to a well-known antioxidant 2,29-methylenebis (4-methyl-6-tert-butyl phenol), CAS# 119-47-1.

Figure 3
You can confirm this species using LC–MS by spiking an authentic reference standard and analyzing according to the method shown in Figure 4. The mass spectral fragmentation behavior, along with the retention time match, provides positive confirmation: the source of the impurity is indeed the rubber closure. The product, therefore, should be monitored to ensure the level of impurity does not pose a toxicological risk to patients.

Figure 4
Example 2
Figure 5 shows an HPLC-UV chromatogram drug product analysis and expanded scale showing an impurity eluted late in the assay. This impurity, unobservable on the full scale, could go undetected. In this case, the drug product is an oral steroid tablet packaged in a high-density polyethylene (HDPE) bottle — at first blush not a likely scenario for migration of container closure species. We converted the method to LC–MS and analyzed using ion APCI MS. It showed a [M+H]+ 183, suggesting a molecular weight of 182 Da (Figure 6).

Figure 5
When attempting to identify unknown species, high-resolution MS can be a quick-screening tool for confirming an empirical formula. In this case, we analyzed the species using a high resolution QTOF LC–MS system (Waters, Milford, Massachusetts) to determine the unknown by exact mass: 183.0801 for the [M+H]+ ion. We proposed the formula C13H11O, with a mass accuracy delta of 4.9 ppm. This species is benzophenone, a common UV inhibitor in label–ink–adhesive systems. The source of benzophenone in the tablets proved to be the secondary label on the HDPE bottle that contained the tablets. The benzophenone was migrating from the labels, through the bottles, and into the solid tablets. Obviously, the manufacturer failed to foresee this migration during the drug product's development, underscoring the need to understand the entire drug development system and implement appropriate levels of control.
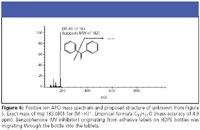
Figure 6
Example 3
The final case study involves an inhalation-solution drug product. Figure 7 shows an LC-UV chromatogram of the API assay. The unknown is eluted at approximately 25 min. In this case, sourcing the impurity was the primary focus. With limited availability of the drug product, we used saline to extract the container closure system in an attempt to source the unknown. Interestingly, however, we observed the unknown in the saline diluent before any interaction with the product's container closure system. Indeed, it was present in the diluent in larger quantities than in the drug product (Figure 8 shows an overlay chromatogram of each). LC–MS analysis demonstrated no ionization in positive ion APCI mode. Negative ion formation appeared to be suppressed by the formic acid buffer used as a substitute for the nonvolatile phosphate buffer.

Figure 7
We used the LC method on a system that collects fractions. The saline diluent was liquid–liquid extracted with methylene chloride, evaporated to dryness, and diluted in a solvent appropriate for reversed-phase LC. We injected the concentrated sample on a preparative LC system and collected multiple fractions of the unknown. The fraction pool was again extracted with methylene chloride and then injected onto a gas chromatography (GC)–MS system.

Figure 8
Unlike APCI analyses, electron ionization (EI) is a well-characterized technique that easily lends itself to library searching. Figure 9 shows a GC–MS total ion chromatogram for the results of this analysis and an EI spectrum for the unknown, along with the reference library match of benzoic acid. Finally, Figure 10 shows a diode array spectrum of a benzoic acid reference standard along with the drug product, showing identical spectra.

Figure 9
For this case study, we obtained quantitative information about the level of benzoic acid present in the drug product. By using an authentic reference standard and API, we obtained a response factor: the amount of benzophenone was approximately 1/15,000 of the API level — about 33 ng in a 2.5-mL inhalation solution ampoule.
Benzoic acid is a common antimicrobial used in many cosmetic applications. Its apparent source was from raw materials used in the production of the drug product.
These studies demonstrate the need to understand materials beyond those in direct contact with the drug product during stability, including those used in conjunction with raw materials, manufacture, and other production.

Figure 10
Conclusions
In its various forms, MS is central technology employed when solving impurity-related problems in the pharmaceutical industry. The examples presented here involve LC–MS-MS, high resolution MS, and GC–EI MS. They show that a systematic approach that adopts the various MS techniques (and used in conjunction with other analytic techniques) can solve challenging issues related to drug product impurities.
References
(1) M.P. Balogh, LCGC 25(6), 554–570 (2007).
(2) D.L. Norwood and F. Qiu, Pharm. Rev. 7 92–99 (2004).
(3) S. Ahuja and K.M. Alsante, Handbook of Isolation and Characterization of Impurities in Pharmaceuticals (Academic Press, San Diego, California, 2003).
(4) K.W. Scott and J. Thompson, Medical Polymers 15–16, 89–110 (2004).
(5) D.R. Jenke, J. Story, and R. Lalani, Int. J. Pharmaceutics 315, 75–92 (2006).
(6) J.S. Kauffman, Pharm. Technol. Anal.Methods S14–S22 (2006).
Michael P. Balogh "MS — The Practical Art" Editor Michael P. Balogh is principal scientist, LC–MS technology development, at Waters Corp. (Milford, Massachusetts); an adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island); and a member of LCGC's editorial advisory board.

Michæl P. Balogh


















New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)