Ionic Liquids by IC–MS
The characteristic composition of ionic liquids (an organic cation or anion and a counterion, in either organic or inorganic form) exhibits unique properties, such as extremely low vapor pressure, excellent thermal stability, electrical conductivity, high polarity, and miscibility with various types of solvents.
Leo (Jinyuan) Wang and William C. Schnute, Dionex Corporation
The characteristic composition of ionic liquids (an organic cation or anion and a counterion, in either organic or inorganic form) exhibits unique properties, such as extremely low vapor pressure, excellent thermal stability, electrical conductivity, high polarity, and miscibility with various types of solvents. Ionic liquids are organic salts with relatively low melting points (below 100 °C) and have been used as solvents in catalysis, organic chemistry, electrochemistry, and separation science.
Here, an ion chromatographic mass spectrometric (IC-MS) profile method for the quantitative determination of anionic ionic liquids and anions in a single chromatographic run is presented.
Experimental
System: ICS-2000 RFIC™ system
Column: IonPac® AS20 and AG20 (2 mm)
Mobile phase: Hydroxide gradient
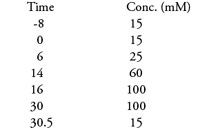
Flow rate: 250 µL/min
Inj. volume: 20 µL
Detection: Suppressed Conductivity and MSQ Plus™ mass spectrometer
Ionization interface: Electrospray Ionization (ESI)
Detection mode: Selected Ion Monitoring (SIM)
Probe temperature: 500 °C
Needle voltage: 1.0 kV
Nebulizer gas: Nitrogen at 85 psi
Results
Figure 1 shows the chromatographic separation of 16 analytes. The SIM chromatograms indicate the differentiation of closely eluted analytes and are shown as single peaks in each SIM channel.

Figure 1
Quantitative experiments provided coefficients of determination (r2 ) > 0.99 for each analyte in the calibration range from low ppb level to 1000 ppb. Method detection limits ranged from 1.04 ppb (tosylate) and 6.13 ppb (sulfate). Three commercially available ionic liquids (dissolved in DI water at 2 mg/mL) were analyzed for impurities by this IC-MS method. Chloride (present in two of the three samples) and bromide (present in all samples) were observed as the major impurities. Other anion impurities such as acetate, methanesulfonate, nitrate, and sulfate were also detected in some or all samples.
IonPac is a registered trademark and RFIC is a trademark of Dionex Corporation.
MSQ Plus is a trademark of Thermo Fisher Scientific, Inc.

Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA
tel. (408)737-0700; fax (408)730-9403
Website: www.dionex.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














