Ion Chromatography for Small Molecule Determination in Clinical and Pharmaceutical Studies
This article presents possible uses of ion chromatography and related techniques combined with various detection methods for clinical and pharmaceutical analysis of common inorganic and organic anions and cations. An overview of achievements in this area from the past 10 years is presented and the most important trends and development perspectives for ion chromatography are described.
In clinical and pharmaceutical research, the application of reliable, reproducible, and the best-available analytical methods and techniques are necessary because the quality of analysis may have a crucial effect on the health and life of patients. The role of inorganic and organic ions, as well as ionizable substances, is very important for health. A very useful instrumental method for this range is ion chromatography (IC) and related techniques. Various ion chromatography methods are used in clinical and pharmaceutical research mainly to determine inorganic anions and cations, metals and metalloids, as well as selected organic compounds. This article presents possible uses of ion chromatography and related techniques combined with various detection methods for clinical and pharmaceutical analysis of common inorganic and organic anions and cations. An overview of achievements in this area from the past 10 years is presented and the most important trends and development perspectives for ion chromatography are described.

Elements and chemical substances, particularly those that may be analyzed using ion chromatography (IC) and related techniques, play an important role in regular human health and life quality. This mainly concerns inorganic anions (F-, Cl- NO3-, PO43-, SO42-) and cations (Na+, K+, Mg2+, Ca2+), selected metals and metalloids (Fe, Cu, Al, Zn, As, Cd, Mn), and organic compounds (amines, carboxylic acids, carbohydrates, peptides, proteins). Depending on the concentration and action time, these substances have a considerable impact on living organisms. Analyses of selected ions in body fluids or tissues can help to understand the causes of various neurological, cardiological, osteoarticular, dermatological, endocrinological, or gynaecological disorders, and their determination is helpful in disease diagnosis, forensic science, and in judicial cases. The use of IC in the pharmaceutical industry is equally important and promising. In recent years, there has been a lot of verified information about the demise of patients caused by consumption of fake medications, medical products, or dietary supplements. It is estimated that half of pharmaceuticals present in the global market are forged (1). The medications and dietary supplements available in the market must be identically effective in terms of therapeutic properties and safety for consumers. Providing a reproducible composition of pharmaceuticals or dietary supplements is related to a very strict quality control of each product series (2).
Ion Chromatography and Related Techniques in Clinical and Pharmaceutical Analysis
IC has been used as a reference method for analyzing anions and cations in water and wastewater for over 40 years (3). Thanks to the introduction of more selective stationary phases in the analytical columns, as well as detection and sample preparation methods, IC applications have been extended to other types of analytes and matrices, including pharmaceutical and biomedical specimens (4). Taking into consideration the separation mechanisms and the resulting types of stationary and mobile phases, it is possible to distinguish IC with or without suppressed conductivity, high perfomance ion exclusion chromatography (HPIEC) (5), and mobile phase ion chromatography (MPIC) (6). HPIEC is mainly used for separation of weak organic and inorganic acids or for group separation of organic and inorganic compounds. MPIC has been applied for determining hydrophobic ions, such as sulphonates, alkaloids, barbiturates, and selected ionic complexes of metals and metalloids. Reversed-phase high performance liquid chromatography (HPLC) (7) and hydrophilic interaction chromatography (HILIC) (8) are also used for analyzing ionizable substances. Ultrahigh-pressure liquid chromatography (UHPLC) technology was originally developed for reversed-phase LC applications, and it is now also available for MPIC and IC due to the availability of 1.7- and 3-μm non-porous particles. It may create a new level of performance in IC, but because of fine particles, the separation quality is improved at the cost of higher pressure. The disadvantage of this technology is that currently there are only a very limited number of commercially available stationary phases.
Simultaneous determination of anions, cations, and neutral species is possible when using a trimodal stationary phase that can provide reserved-phase, anion-exchange, and cationâexchange retention mechanism. Taking into consideration problems with the selectivity of analyte separations, particularly in complex matrices samples, an interesting solution is multidimensional IC. It is based on linking two individual chromatographic systems in such a way that a section of the effluent of the first system after passing the detector is transferred into the second chromatographic system (9,10).
The detection methods used in IC and related techniques can be divided into direct and indirect methods, or into electrochemical (conductometric and amperometric detection) and spectroscopic (UV–vis, mass spectrometry [MS]) techniques (11). Conductometric detection is used most often, but it does not allow for determining substances with values of pK > 7. In the range of inorganic analysis, IC with conductivity detection was suggested as a reference method for determining Na, K, Ca, and Mg ions in the blood serum as early as 1997 (12). Contactless conductivity measurements are another option; they have been employed for the detection of inorganic or small organic ions in conventional capillary electrophoresis, and less often in microchip electrophoresis (13). A very useful detection method, specific for compounds, such as, carbohydrates, amino acids, alditols, glycols, and alcohols, is pulsed amperometric detection (PAD). The application of cation-exchange chromatography (CEC) coupled with integrated pulsed amperometry in clinical tests was first described by Cole and Evrovski (14) in 1997. A PAD detector can be used for the determination of iodide ions in healthy and pathological human thyroids, urine, and serum samples, in addition to the determination of common inorganic ions in serum (15). Other detectors used for organic substances determination are evaporative light scattering detectors (ELSDs) (16) and charged aerosol detectors (CADs) (17). Instead of measuring the intensity of radiation absorbed by analytes present in the eluate, an ELSD measures the radiation dispersed on the particles of the aerosol formed by the analytes (18). CAD offers high sensitivity, broad dynamic measurement range, and result repeatability (19). For metal and metalloids analysis, UV–vis detection and atomic absorption spectrometry (AAS), inductively coupled plasma optical emission spectrometry (ICP-OES), and inductively coupled plasma–mass spectrometry (ICP-MS) (20) offer advantages such as speed, good precision, and repeatability. Their disadvantages include lack of differentiation between metals occurring at different oxidation states. For that reason, different separation and detection methods are coupled in the form of hyphenated techniques (21). The most popular IC-based hyphenated techniques are IC-ICP-MS and IC-MS (22). They offer great possibilities and their main advantages include extremely low limits of detection and quantification, and very good precision and repeatability of determinations. However, they have some limitations, such as high price and complexity of the apparatus, as well as requiring an understanding of analytical methodologies and instruments. At present, most studies concern hyphenated techniques based on mass spectrometry, including those dedicated to metabolite analyses in clinical and pharmaceutical samples (23).
Sample Preparation Methods
The opportunities offered by IC and its varieties for pharmaceutical and clinical research are related to progress in the development of sample preparation methods. These kinds of samples usually require time-consuming preparation procedures before the chromatographic analysis. The risk is related to various oxidation, reduction, complexation, precipitation, and bio- and photochemical processes that may occur in the sample between the sampling and the analyte determination (24). As a result of the sample state of matter, two groups of preparation methods can be distinguished. The first one concerns liquid samples and includes filtration, dilution, pH changes, derivatization, liquid–liquid extraction (LLE), solid-phase extraction (SPE), and membrane techniques (25). The other is used for solid samples and encompasses drying, homogenization, extraction/elution, etching, or incineration. Dense solid samples analyzed with IC require turning the analytes into a solution. Such analytical systems are collectively known as combustion ion chromatography (CIC). Its main advantages are: extension of IC applications for analyzing solid samples in an online mode; full automation and control of the incineration process; application of one software type for the entire analytical procedure; possible quick exchange of attachments for solid and liquid sample analyses; and simple calibration (26).
Examples of IC Applications in Clinical and Pharmaceutical Studies of Small Molecules
One year after the official establishment of IC, the first study on its uses in clinical analysis was published (27). Bhattacharyya and Rohrer (28) described examples of IC applications in tests of pharmaceutical products and biomedical samples. In 2011, Jenke (29) discussed IC applications for clinical research and the pharmaceutical industry in an overview study. IC is an important analytical method for the analysis of pharmacopoeia grades of water used in pharmaceutical industry (30).
Very popular IC applications in clinical analysis are determining ions and other substances in perspiration. Perspiration mainly consists of water (~ 99%) with small amounts of nitrogen compounds (such as amino acids and urea), K+, Na+, and Cl- ions, and various metabolites and xenobiotics. When a disease occurs, perspiration may be a source of information on biomarkers of various diseases, including schizophrenia, cystic fibrosis, diabetes, or cancer (31). Thanks to its characteristics, perspiration analysis is a quick and simple process when compared to analyzing other body fluids. This particularly concerns blood because its collection has an invasive character and is related to a higher risk of infection. At present, progress in the miniaturization of measurement apparatus allows for perspiration or saliva tests to be undertaken without a visit to a clinic or hospital.
IC is a decisive analytical tool for solving some analytical problems in the field of nephrology. Many methods used in the past for determining oxalates in the urine of patients suffering from kidney stones, such as permanganometry (after oxalate oxidation to CO2) or spectrophotometry (after oxalate reduction to glycoxylic or glycolic acid) are time- and work-consuming. Another interesting example is analyzing the ion composition of fluids for peritoneal dialysis and dialysates themselves. The appropriate concentrations of anions and cations and mutual concentrations determine the dialysis correctness and efficiency. Figure 1 shows chromatograms of anions in the dialysis fluids and dialysates sampled from the patient treated with peritoneal dialysis (32).
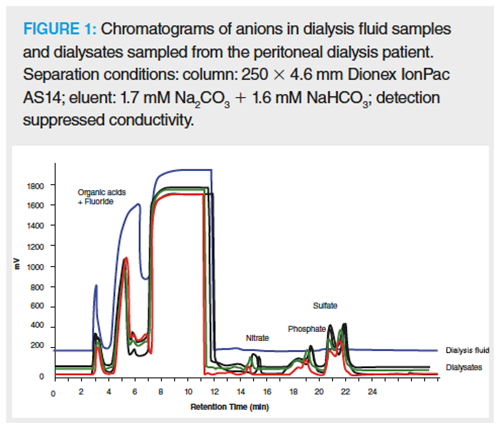
Research into the biomarkers of many conditions has contributed to the extension of IC applications for analyses of body fluids (blood, urine) (33) or tissue samples (thyroid) (34). The studies of plasma samples in patients with alcoholic liver cirrhosis showed a significant correlation between many heavy metals and the disease (35). The research into metals in hair samples with IC demonstrated a significant correlation between Zn, Pb, and depression (36). Other IC applications in clinical analysis include determinations of homocysteine (risk factor in coronary artery disease), methionine together with other amino acids present in the plasma, or catecholamines (including noradrenaline, dopamine) in urine. Smith et al. (37) developed a method based on IC whose advantages included the ability to determine the diversity of physiologically important anions in one cycle. Carbohydrate analyses in bodily fluid samples are more and more often conducted with high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The clinical application of this method concerns, among others, monitoring of the irregular functioning of the intestines during the HIV infection (38). An important application is determination of deoxy-2-[18]fluoro-D-glucose, which is one of the most widely used radiopharmaceuticals for positron emission tomography studies (39).
Among many pharmaceutical products, there are some that can be determined directly by IC methods. One example is the group of bisphosphonates that are used to treat bone disorders including osteoporosis, such as clodronate or waterâsoluble vitamins. As a result of similar properties and low separation selectivity a serious problem in pharmaceutical analysis is the analysis of aliphatic amines. A typical example for the significance of aliphatic amines is the analysis of amyloamine and tert-butylamine. Next, for the analysis of aliphatic quaternary ammonium compounds, cationâexchange chromatography with conductivity detection can be applied. IC with conductometric detection is used for determining morpholine pollutants in molsidomine (used in prophylaxis and treatment of angina pectoris and cardiac insufficiency). An example chromatogram is presented in Figure 2.
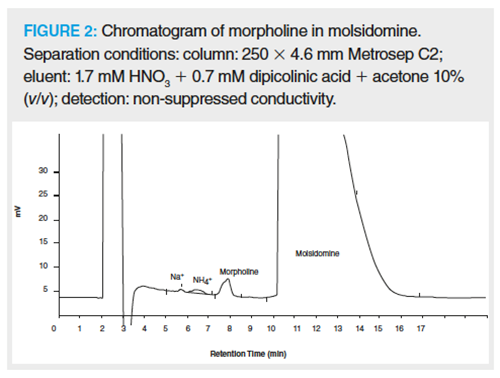
In recent years, the number of monographs discussing research procedures based on IC has been on the rise. The US Pharmacopeia32–National Formulary 27 edition contained two chapters on IC (345 and 1065), and four chapters describing measurement methods based on IC (1045; 1052; 1055; 1086). At present, the document contains over 110 monographs using one or more research IC-based procedures.
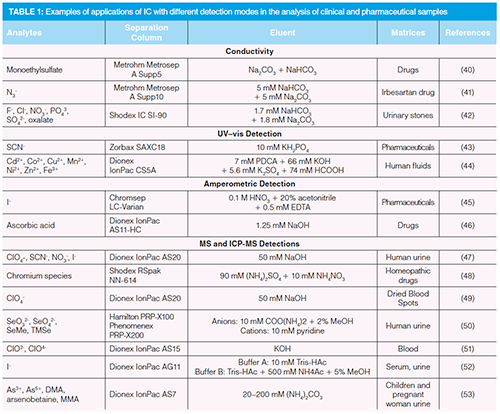
Table 1 presents examples from the literature over the past 10 years concerning the applications of IC and its varieties in the clinical and pharmaceutical research grouped according to the used detection mode (40–53).
Conclusions
IC and related techniques have been extensively employed in clinical and pharmaceutical research. The most important advantages in IC are: the simultaneous determination of several ions in a short time; the small sample volume necessary for analyses; the possibility of using different detectors; simultaneous separation of anions and cations, or organic and inorganic ions; separation of ions of the same element at different oxidation states (speciation analysis); full automatization; and safety and low exploitation costs (green chemistry). Like all methods, IC has some drawbacks. The most important are the limited selectivity in complex matrices and the time-consuming and labour-intensive methods for most clinical and pharmaceutical samples.
Future Trends and Perspectives
The future of clinical and pharmaceutical applications of IC and related techniques will involve interdisciplinary studies and in-depth speciation analyses suitable for evaluating the analysis of typical and non-typical analytes. Furthermore, interesting possibilities still exist in the areas of ultrahigh speed separations, tools for computer-assisted method development, multidimensional separations, and miniaturization. The challenges in IC are also related to increasing the use of these techniques in molecular biology and genetics research (genomics, proteomics, metabolomics, transcriptomics). Certainly, the interest from the clinical studies and pharmaceutical industry in IC and related techniques is growing and is expected to increase steadily in the future (54).
References
- H. Rebiere, P. Guinot, D. Chauvey, and C. Brenier, J. Pharm. Biomed. Anal.142, 286–306 (2017).
- R. Michalski, Mini Rev. Med. Chem.14(10), 862–872 (2014).
- R. Michalski, Crit. Rev. Anal. Chem. 36(2), 107–127 (2006).
- J. Weiss, Handbook of Ion Chromatography, Third, completely revised and updated edition (Wiley-VCH Verlag GmBH & Co. KGaA, Weinheim, Germany, 2008)
- J.S. Fritz, J. Chromatogr. A546, 111–118 (1991).
- M.T.W. Hearn, Ion-pair chromatography: theory and biological and pharmaceutical applications (Marcel Dekker Inc, New York, New York, USA,1985).
- M.C. Gennaro and S. Angelino, J. Chromatogr. A789(1), 181–194 (1997).
- B. Buszewski and S. Noga, Anal. Bioanal. Chem. 402(1), 231–247 (2012).
- C.J. Venkatramani, Advances in Pharmaceutical Analysis, LCGC Europe Special Issue31(s10), 22–29 (2018).
- M. Iguiniz and S. Heinisch, J. Pharm. Biomed. Anal.145, 482–503 (2017).
- P.R. Haddad, P.N. Nesterenko, and W. Buchberger, J. Chromatogr. A1184(1), 456–473 (2008).
- L. Thienpont, J.E Van Nuwenborg, and D. Stöckl, J. Chromatogr. A789, 557–568 (1997).
- P. KubáÅ and P.C. Hauser, Electrophoresis40(1), 124–139 (2019).
- D.E.C. Cole and J. Evrovski, J. Chromatogr. A789(1), 221–232 (1997).
- A. BÅażewicz et al., J. Chromatogr. B879(9), 573–578 (2011).
- N. Vervoort, D. Daemen, and G. Torok, J. Chromatogr. A1189(1–2), 92–100 (2008).
- L.-E. Magnusson, D.S. Risley, and J.A. Koropchak, J. Chromatogr. A1421, 68–81 (2015).
- E. Dvorackova, M. Snoblova, and P. Hrdlicka, J. Sep. Sci.37(4), 323–337 (2014).
- Z. Huang et al., J. Pharm. Biomed. Anal.50(5), 809–814 (2009).
- R. Michalski, Crit. Rev. Anal. Chem. 39(4), 230–250 (2009).
- R. Michalski, M. JabÅonska, S. Szopa, and A. Åyko, Crit. Rev. Anal. Chem.41(2), 133–150 (2011).
- R. Michalski, Application of IC-MS and IC-ICP-MS in Environmental Research (John Wiley & Sons, Inc., Hoboken, New Jersey, USA, 2016).
- N. Iwamoto and T. Shimada, Pharmacol. Ther.185, 147–154 (2018).
- P.R. Haddad, P. Doble, and M. Macka, J. Chromatogr. A856(1–2), 145–177 (1999).
- W. Frenzel and R. Michalski, in Application of IC-MS and IC-ICP-MS in Environmental Research, R. Michalski, Ed. (John Wiley & Sons, Inc., Hoboken, New Jersey, USA, 2016), pp. 210–266
- J.-M. Liu, C. Liu, G.-Z. Fang, and S. Wang, RSC Adv. 5, 58713–58726 (2015).
- C. Anderson, Clin. Chem.22(9), 1424–1426 (1976).
- L. Bhattacharyya, in Applications of Ion Chromatography for Pharmaceutical and Biological Products, L. Bhattacharyya, Ed. (John Wiley & Sons, Inc., Hoboken, New Jersey, USA, 2012), pp. 135–157.
- D. Jenke, J. Chromatogr. Sci.49(7), 524–539 (2011).
- J.P. Waterworth, J. Chromatogr. A770(1–2), 99–104 (1997).
- A. Mena-Bravo and M.D.L. de Castro, J. Pharm. Biomed. Anal. 90, 139–147 (2014).
- R. Michalski and A. Lyko, J. Liq. Chromatogr. Relat. Technol. 39, 96–103 (2015).
- A. Blazewicz, M. Klatka, A. Astel, M. Partyka, and R. Kocjan, Biol. Trace Elem. Res.155(2), 190–200 (2013).
- A. Blazewicz et al., J. Chromatogr. B, Anal. Technol. Biomed. Life Sci. 878(1), 34–38 (2010).
- A. BÅażewicz et al., Biomed Res. Int.2017, 1–10 (2017).
- A. Prystupa et al., Int. J. Environ. Res. Public Health13(6), 582–592 (2016).
- R.E. Smith et al., J. Chromatogr. A439(1), 83–92 (1988)
- D. Rojo, C. Barbas, and F.J. Ruperez, Bioanalysis4(10), 1235–1243 (2012).
- P. Hajos, D. Lukacs, E. Farsang, and K. Horvath, J. Chromatogr. Sci. 54(10), 1752–1760 (2016).
- S.J. Prasanna, H.K. Sharma, K. Mukkanti, M. Sivakumaran, K.S.R.P. Kumar, and V.J. Kumar, J. Pharm. Biomed. Anal.50, 1065–1069 (2009).
- N.H. Subramanian, P. Manigandan, R.G. Jeevan, and G. Radhakrishnan, J. Chromatogr. Sci.47(7), 540–544 (2009).
- E. Saussereau, J.-P. Goulle, and C. Lacroix, J. Anal. Toxicol. 31(7), 383–387 (2007).
- C. Fernandes, R.S. Leite, and F.M. Lancas, J. Chromatogr. Sci.45(5), 236–241 (2007).
- P. Zakaria et al., J. Chromatogr. A1216(38), 6600–6610 (2009).
- T.K. Malongo et al., Talanta76(3), 540–547 (2008).
- M. Sahin, L. Ozcan, B. Usta, and Y. Sahin, Biosens. Bioelectron.24(12), 3492–3497 (2009).
- L. Valentin-Blasini, B.C. Blount, and A. Delinsky, J. Chromatogr. A1155(1), 40–46 (2007).
- H. Hagendorfer and W. Goessler, Talanta76(3), 656–661 (2008).
- S.M. Otero-Santos, A.D. Delinsky, L. Valentin-Blasini, J. Schiffer, and B.C. Blount, Anal. Chem. 81(5), 1931–1936 (2009).
- T. Jäger, H. Drexler, and T. Göen, J. Anal. At. Spectrom. 28, 1402–1409 (2013).
- A. Schwan, R. Martin, and W. Goessler, Anal. Methods7, 9198–9205 (2015).
- B. Michalke and H. Witte, J. Trace Elem. Med. Biol. 29, 63–68 (2015).
- A.J. Signes-Pastor et al., Expo. Heal. 9(2), 105–111 (2017).
- G. Cudiama, LCGC North America36(1), 12 (2018).
Rajmund Michalski works in the Institute of Environmental Engineering at the Polish Academy of Science in Zabrze (Poland) since 1988. His research interests are ion chromatography (sample preparation, columns, eluents, detectors, and applications) and application of hyphenated techniques (IC-ICP-MS, IC-MS) in species analysis (inorganic disinfection byproducts, metal/metalloids ions). He has authored books, manuscripts, and over 170 publications about the applications of ion chromatography in environmental and food samples.
Anna BÅażewicz is an associate professor at the Medical University of Lublin where she is the head of the Laboratory of Instrumental Analysis and currently coordinates eight interdisciplinary, international, non-commercial research projects. Her research interests include mainly new applications of chromatographic techniques, clinical, food and environmental chemistry, bioanalytics, and pathobiochemistry.
Joanna KoÅczyk is a researcher and academic teacher in the Institute of Chemistry of Jan Dlugosz University in Czestochowa. Her scientific interests include application of chromatographic (HPLC and IC) and spectroscopic (AAS, UV–vis) methods for quantitative and qualitative analysis of environmental, pharmaceutical, and food samples.
Katarzyna Krupa is a doctoral candidate in the Department of Analytical Chemistry at the Medical University of Lublin. Her primary research focus is the determination of heavy metals in various clinical samples, ion chromatography, and mass spectrometry.
PrzemysÅaw NiziÅski is a doctoral candidate in the Department of Analytical Chemistry at the Medical University of Lublin. His research interest include environmental toxicology, especially endocrine disruptors and heavy metals pollution, ion chromatography, and sample preparation techniques.
Best of the Week: Food Analysis, Chemical Migration in Plastic Bottles, STEM Researcher of the Year
December 20th 2024Top articles published this week include the launch of our “From Lab to Table” content series, a Q&A interview about using liquid chromatography–high-resolution mass spectrometry (LC–HRMS) to assess chemical hazards in plastic bottles, and a piece recognizing Brett Paull for being named Tasmanian STEM Researcher of the Year.
Using LC-MS/MS to Measure Testosterone in Dried Blood Spots
December 19th 2024Testosterone measurements are typically performed using serum or plasma, but this presents several logistical challenges, especially for sample collection, storage, and transport. In a recently published article, Yehudah Gruenstein of the University of Miami explored key insights gained from dried blood spot assay validation for testosterone measurement.
Determination of Pharmaceuticals by Capillary HPLC-MS/MS (Dec 2024)
December 19th 2024This application note demonstrates the use of a compact portable capillary liquid chromatograph, the Axcend Focus LC, coupled to an Agilent Ultivo triple quadrupole mass spectrometer for quantitative analysis of pharmaceutical drugs in model aqueous samples.