Improving Selectivity in the Chromatographic Analysis of Monoclonal Antibodies (mAbs) Through the Use of Multi-Isocratic Elution Mode
When characterizing monoclonal antibodies (mAbs) with reversed-phase liquid chromatography (RPLC), it is often challenging to attain sufficient selectivity between mAbs and their related variants. A new strategy, referred to as multi-isocratic elution mode, has recently been developed. It is based on setting a series of consecutive isocratic steps and very short steep gradient segments at solute elution. This elution mode offers several advantages compared to the usually applied linear gradient mode. Large biomolecules can benefit the most because of their “on/off” elution behavior. Arbitrary selectivity can be set between closely related protein variants while maintaining sharp peaks because of the strong band compression effects occurring at elution within the steep gradient segments.
Monoclonal antibodies (mAbs) are considered one of the most important classes of therapeutic molecules for treating various diseases. These biopharmaceutical products are highly complex and inherently heterogeneous, making them challenging to characterize. Even a minor chemical modification (such as oxidation, deamidation, glycation, and so forth) in the protein structure could strongly affect the product efficacy or toxicity. Therefore, the presence of minor isoforms (also known as variants) has to be characterized using powerful chromatographic methods in combination with mass spectrometry (MS) (1). Reversed-phase liquid chromatography (RPLC) is a robust, reliable, and versatile chromatographic approach, and it is widely used today for characterizing protein biopharmaceuticals. RPLC offers high kinetic performance and straightforward compatibility with MS, but the technique has also shown some limitations, which are described in the literature (2). An important constraint of RPLC is the limited selectivity when characterizing large molecules.
Tuning Selectivity in Reversed-Phase LC Mode
When analyzing protein biopharmaceuticals in RPLC, the nature of the stationary phase has a very limited impact on selectivity and retention except when using exotic stationary phase chemistry (3). Selectivity is mostly driven by the mobile phase. Unfortunately, the flexibility in terms of mobile phase additives is extremely restricted. Trifluoroacetic acid (TFA) is often required to obtain symmetrical and sharp peaks for mAbs (4). In addition, the impact of organic modifier nature (acetonitrile, methanol, and isopropanol) on selectivity is also quite limited (5). Because of these considerations, it is clear that a modifying gradient elution profile should be the most promising solution to tune selectivity.
The Particular Elution Behavior of Proteins in Reversed-Phase LC Mode
It is established that large molecules, such as mAbs, display specific elution behavior. Their retention is extremely sensitive to the mobile phase composition. A very minor change in eluent strength can result in the complete release of the solute from the column. This particular retention behavior has been described as an “on/off” or “bind and elute” mechanism (6). Compared to the multiple-step partitioning process that is observed with small molecules in RPLC mode, only a very limited number of adsorption-desorption steps are observed with large molecules. To better explain this retention mechanism, the retention factor of a large solute is nearly infinite in a weak eluent; thus, the protein species do not migrate along the column (adsorbed at the column inlet, thus corresponding to the “on” state). Then, only a small increase of eluent strength (for example, a 1% increase of organic modifier content shifts the retention by a factor of 10 for a mAb [7]), resulting in a huge drop of retention factor and the protein migrating towards the column outlet without any further interactions (this corresponds to the “off” state).
Based on this behavior, it is clear that gradient elution is mandatory to elute various protein species contained within one given sample and to control the retention of large biomolecules.
Multi-Isocratic Elution Mode for Proteins
In practice, a simple linear gradient is applied for analyzing proteins under RPLC conditions because it is a very simple procedure, highly repeatable (poorly affected by flow pumping precision and system volumes), and easy to transfer between instruments. Some other types of gradients (concave or convex) have also been successfully applied to mAbs analysis, but remain rarely used (8).
Alternatively, to benefit from the on/off behavior, we have developed a strategy of combining isocratic (binding) steps and very short steep gradient (eluting) segments at protein elution composition (7). Such a multi-step elution mode allows us to tune selectivity as desired while maintaining sharp peaks because of the strong band compression effect observed with proteins. If desired, extremely high selectivity can be reached because the selectivity will exclusively be determined by the duration of a given isocratic segment.
Figure 1 shows a schematic view of the multi-isocratic elution mode for proteins. On the top panel of Figure 1, a linear gradient was applied for separating various protein species (subunit species of a mAb, all of ~25 kDa). In this chromatogram, various segments (#1 to #4) were identified and the corresponding eluent compositions could be easily calculated. As shown at the bottom panel of Figure 1, when setting the eluent composition to 27%, all the protein species bind at the column inlet. Then, when increasing the eluent strength to 30%, only the first peak (least retained) was eluted while the two other peaks were highly retained at the column inlet. By increasing the eluent composition to 32.5%, the second compound will elute while the third one is still strongly bound to the stationary phase. Finally, the last compound can be eluted by setting 37% B.
FIGURE 1: Schematic representation of the elution of protein species when running (a) linear gradient, and (b) multi-isocratic elution mode. Adapted from (7) with permission from the American Chemical Society.
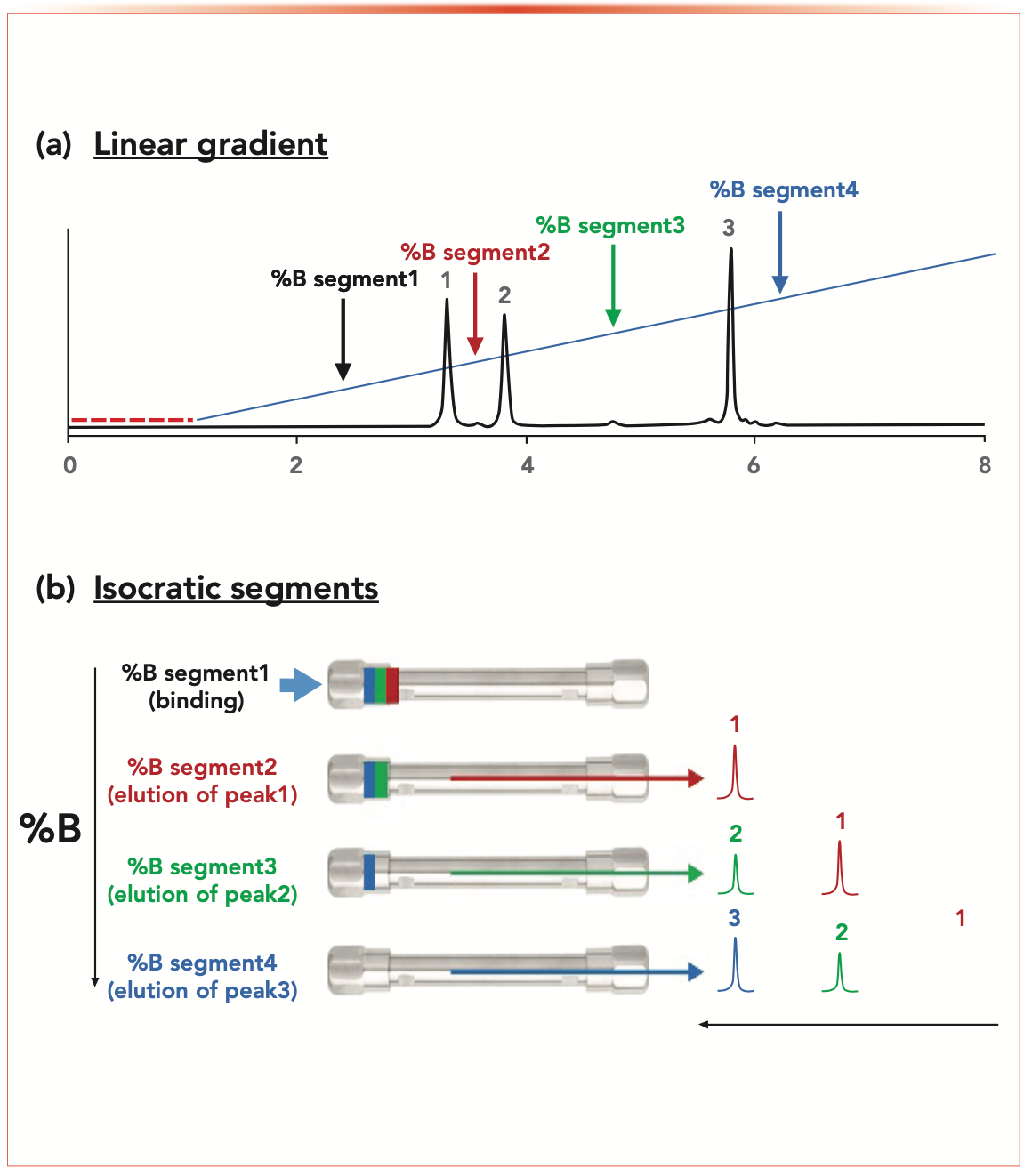
Much higher selectivity is expected with a multi-isocratic segment elution program compared to any linear gradient simply by setting the length of each isocratic segment appropriately. Tuning the segment duration will only affect the retention of the next eluting peak and will have a negligible effect on later eluting peaks.
Applications of the Procedure to Real Samples
Figure 2 highlights two relevant applications of the multi-isocratic elution mode for analyzing real mAb samples.
FIGURE 2: Separation of (a) daratumumab subunits (reduced sample), and (b) separation of intact antibody (atezolizumab) variants using linear gradient and multi-isocratic elution mode. Here, the selectivity was arbitrarily changed by adjusting the length of the isocratic segment between the two peaks. Adapted from (7) with permission from the American Chemical Society.
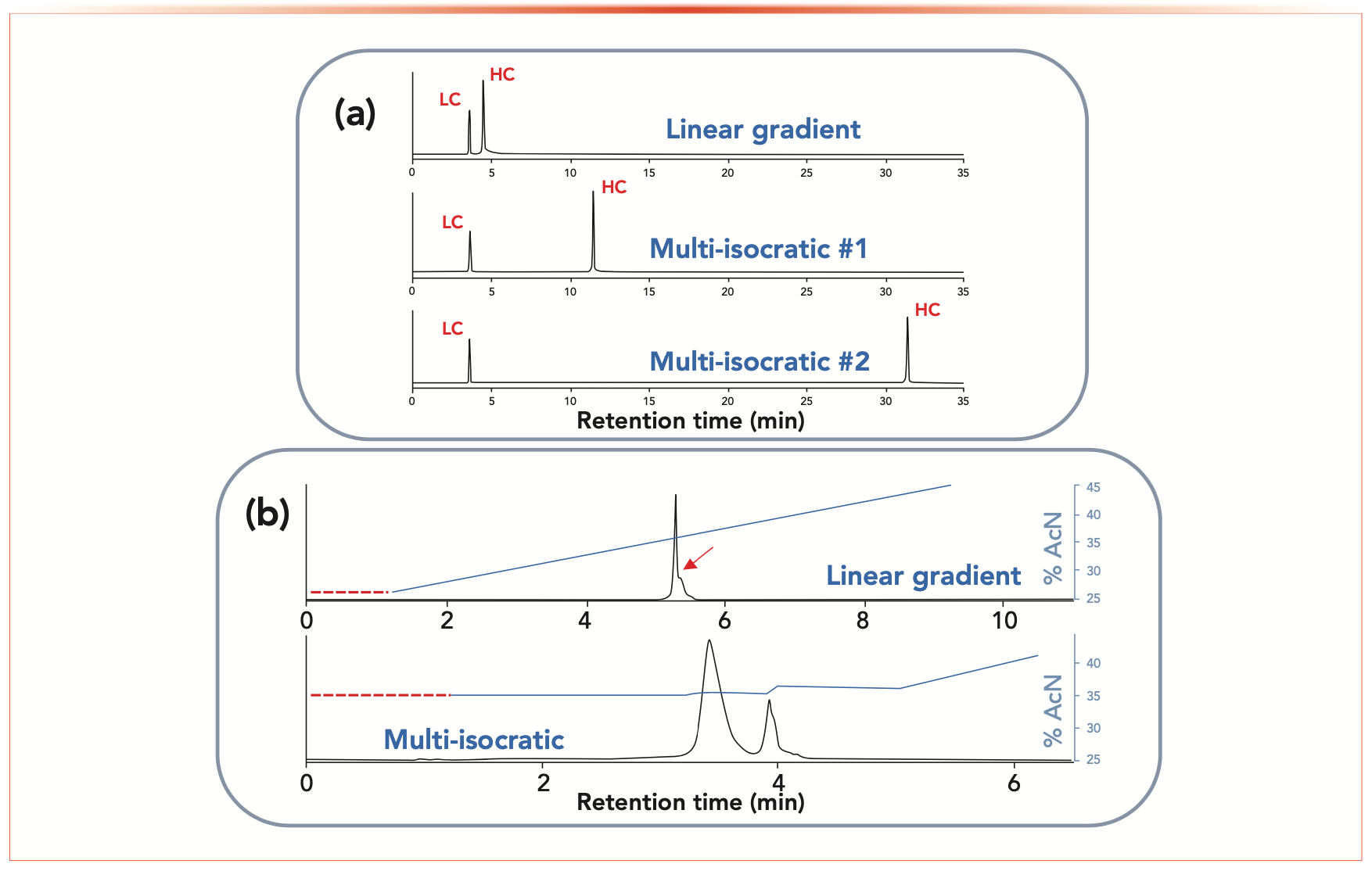
Figure 2a shows the analysis of a reduced daratumumab sample. Chemical reduction with dithiothreitol (DTT) produces two peaks, one for the heavy chain (HC) of 50 kDa and another one for the light chain (LC) of 25 kDa. The separation between LC and HC was satisfactory using a linear gradient (first chromatogram in Figure 2a), but much higher selectivity was achieved by extending the length of the isocratic segment between the two peaks (an isocratic step of 8 and 28 min was included between the elution of the two peaks for the second and third chromatograms, respectively). In addition, the width of the HC peak was not affected when extending the isocratic time duration. This example also highlights the possibility of achieving high selectivity between protein species under RPLC conditions (see the third chromatogram on Figure 2a).
Figure 2b shows the analysis of an intact atezolizumab sample of approximately 150 kDa. When applying a linear gradient, only a shoulder can be observed at the right bottom of the main peak and this second peak is not sufficiently resolved from the main isoform. These two peaks cannot be separated whatever the steepness of a linear gradient. However, when applying multi-isocratic elution mode, the two peaks can be well resolved, as illustrated in Figure 2b. Nevertheless, it is important to mention that the two protein species have a very close chromatographic behavior (very similar retention model parameters). Therefore, this example shows the limits of what can be done with this approach.
Perspectives
With the multi-isocratic approach, uniform peak distribution equidistant band spacing) and significantly higher resolution can be achieved compared to common linear, multi-linear, or any non-linear gradients.
However, such a procedure may be insufficient in improving resolution when the protein species have similar retention properties. Therefore, we have demonstrated that a steep negative gradient segment between the eluting and non-eluting segments can be added to further improve selectivity (9). In practice, this strategy is more difficult to implement than the multi-isocratic approach but can be particularly attractive.
All the results presented here were obtained under RPLC conditions. However, it is very important to notice that the multi-isocratic approach can also be valuable in other modes of chromatography such as ion exchange chromatography (IEC), hydrophobic interaction chromatography (HIC), and hydrophilic interaction chromatography (HILIC) (10).
References
(1) N. Nupur, S. Joshi, D. Guillarme, and A.S. Rathore, Front. Bioeng. Biotechnol. In press (2022). DOI: 10.3389/fbioe.2022.832059
(2) V. D’Atri, A. Murisier, S. Fekete, J.L. Veuthey, and D. Guillarme, TrAC Trends Anal. Chem. 130, 115962 (2020). DOI: 10.1016/j.trac.2020.115962
(3) S. Fekete, S. Rudaz, J. Fekete, and D. Guillarme, J. Pharm. Biomed. Anal. 70, 158–168 (2012). DOI: 10.1016/j.jpba.2012.06.021
(4) H. Lardeux, B.L. Duivelshof, O. Colas, A. Beck, D.V. McCalley, D. Guillarme, and V. D’Atri, Anal. Chim. Acta 1156, 338347 (2021). DOI: 10.1016/j.aca.2021.338347
(5) S. Fekete, A. Murisier, A. Beck, J. Lawhorn, H. Ritchie, B. Boyes, and D. Guillarme, J. Chromatogr. A 1642, 462050 (2021). DOI: 10.1016/j.chroma.2021.462050
(6) L.R. Snyder, M.A. Stadalius, and M.A. Quarry, Anal. Chem. 55(14), 1412A–1430A (1983). DOI: 10.1021/ac00264a001
(7) S. Fekete, A. Beck, J.L. Veuthey, and D. Guillarme, Anal. Chem. 91(20), 12954–12961 (2019). DOI: 10.1021/acs.analchem.9b03005
(8) V.S. Joshi, V. Kumar, and A.S. Rathore, J. Chromatogr. Sci. 53(3), 417–423 (2015). DOI: 10.1093/chromsci/bmu222
(9) S. Fekete, A. Murisier, J.M. Nguyen, M.A. Lauber, and D. Guillarme, J. Chromatogr. A 1635, 461743 (2021). DOI: 10.1016/j.chroma.2020.461743
(10) S.L. Khatwani, A. Pavlova, and Z. Pirot, Mol. Ther. Methods Clin. Dev. 21, 548–558 (2021). DOI: 10.1016/j.omtm.2021.04.003
Thomas Bouvarel is with the Institute of Pharmaceutical Sciences of Western Switzerland (ISPSO) at the University of Geneva, in Geneva, Switzerland.

Szabolcs Fekete is with Waters Corporation in Milford, Massachusetts.

Davy Guillarme is with the School of Pharmaceutical Sciences, at the University of Geneva, in Geneva, Switzerland. Direct correspondence to: davy.guillarme@unige.ch

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.














