Identifying and Collecting Weak UV Absorbance and Low Level Peaks of Interest From a Complex Mixture Using Conditional Logic Purification on Mass and UV Signals
Purification of active pharmaceutical ingredients (APIs) often yields high numbers of fractions, leading to a bottleneck of fractions to dry-down and subsequently analyze. UV-based fraction collection based on slope or threshold values offers some specificity and reduction in the number of fractions collected compared to basic time- or volume-based collection.
Purification of active pharmaceutical ingredients (APIs) often yields high numbers of fractions, leading to a bottleneck of fractions to dry-down and subsequently analyze. UV-based fraction collection based on slope or threshold values offers some specificity and reduction in the number of fractions collected compared to basic time- or volume-based collection. LC–MS-based purification provides the highest specificity. This application will demonstrate the improvement in specificity offered by LC–MS-based purification when compared to traditional UV alone.
Mass-based purification offers enhanced selectivity for purifying APIs often resulting in higher purity and fewer fractions to process. Typical synthesis steps and reactions often lead to impurities and partial products. In some cases, the desired product will not be the predominant compound detected. This represents challenges in purification with traditional UV-triggered fraction collection. Applying conditional logic collection parameters (e.g., AND) to both UV AND target mass signals, selectivity can be dramatically improved to where only the APIs of interest are collected. In addition, there is confidence of identity provided by the full scan collaterally generated mass spectral data.
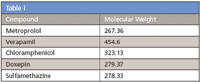
Table I
In this study, API purification was simulated using a mixture of several pharmaceuticals at varying concentration levels. The compound of interest representing the API has a low, relative percent area compared to other compounds present in the same sample solution.

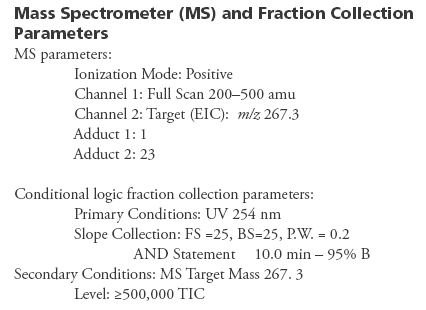
Results
Performing fraction collection using only UV detection and peak slope collection produces seven different fractions. Each of these would be subdivided into two or three fractions resulting in ~20 total fractions consisting of 15 or 20 mL volumes. This is shown below using Fraction Simulation within TRILUTION LC.
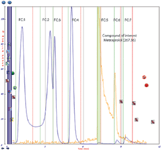
Figure 1: Fraction simulation using UV and Slope Collection mode
The simulated fraction collection for the API of interest (metroprolol) in this demonstration is a minor component when compared to the other compounds (of non-interest) present. The same compound mixture was purified with conditional logic collection utilizing both a UV wavelength of 254 nm AND a target mass of 267.36 (metroprolol). The metroprolol target mass channel showed tailing. As a result, the next UV peak was eluted while the target mass of 268.2 [M+H] was still present, which caused a second fraction to be collected. The gradient conditions could be modified to increase the separation and prevent the second fraction. Increasing the make-up flow rate to 0.5 mL/min was attempted to reduce the amount of tailing with minimal improvement.
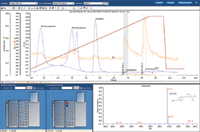
Figure 2: Purification using Conditional Logic Fraction Collection based on UV and MS
The metroprolol peak (see Figure 3, peak at 6.823 minutes) only represented 0.125% of the total peak area. Purification using Conditional Logic Fraction Collection based on UV and MS signals, the number of fractions were reduced to only two fractions.

Figure 3: Sample Table
Conclusions
UV-based purification is an effective means of obtaining pure compound of interest; however, there are limitations with compound specificity. These limitations lead to large numbers of fractions obtained, many of which do not contain the compound of interest. Post fraction collection processing includes dry down, and often re-injection onto LC–MS for compound verification. Combining UV and LC–MS for purification provides the capabilities to reduce the number of fractions collected and thus reduction in post processing time as a result of positive confirmation of the compound at collection.
Conditional logic (UV AND MS signals) properly applied to a complex mixture is shown to provide very good selectivity for fraction collection even if the target compound is present at very low relative amounts.
Gilson, Inc.
3000 Paramenter St., Middleton, WI 53562
tel. (800) 445-7661; fax (608) 831-4451
Website: www.gilson.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














