Highly Efficient Separation of Bath Salts Using Hydrophilic Interaction Chromatography (HILIC)
According to law enforcement and health officials, "bath salts" are powerful synthetic stimulants, designed to be comparable in effect to cocaine or methamphetamine, carrying similar risks. Unlike cocaine or methamphetamine, these stimulants are currently legal in many parts of the United States and available at a low cost in many convenience stores and head shops.
According to law enforcement and health officials, "bath salts" are powerful synthetic stimulants, designed to be comparable in effect to cocaine or methamphetamine, carrying similar risks. Unlike cocaine or methamphetamine, these stimulants are currently legal in many parts of the United States and available at a low cost in many convenience stores and head shops.
Recognition of the potential risks has prompted many regions to ban the sale of bath salts, leading to the need for analytical methods to meet the challenges associated with regulation enforcement. In addition, there exists a need for fast and reliable methods for use in health care facilities.
Experimental
Figure 1 shows the structures and selected physiochemical properties of two bath salt components. These compounds are representative of a class of polar, basic molecules. Such analytes are often difficult to retain and separate using standard reversed-phase chromatography. Several of the analytes exhibit similar solubility properties as indicated by the comparable predicted LogD values. Several compounds also show almost equivalent ionization constants as well. These efforts, therefore, focused on the use of HILIC chromatographic techniques to obtain efficient retention and selectivity.
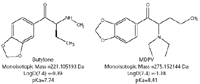
Figure 1: Structures and physiochemical properties of two representative bath salts (1,2).
Figure 2 shows a highly efficient LC–MS separation of six chosen analytes using Ascentis® Express HILIC. The HILIC phase exhibits partition and ion-exchange characteristics, making it highly suitable for separation of polar, weak bases. Attempts at obtaining equivalent separation using conventional C18 or alternative reversed-phase chemistries have been unsuccessful to date. In addition, of the HILIC phases explored, only Ascentis Express HILIC provided baseline resolution of all six analytes.
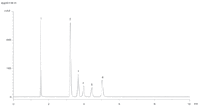
Figure 2: Analysis of illicit bath salts on Ascentis Express HILIC. System: Agilent 1200SL Rapid Resolution, 6210 TOF, Column: Ascentis Express HILIC, 5 cm à 2.1 mm I.D., 2.7 µm (53934-U), Mobile Phase: 5 mM ammonium formate (5:95 water:acetonitrile), Flow Rate: 0.6 mL/min., Temp.: 35 °C, System Pressure: 88 bar, Inj.: 1 µL, MS Detection: ESI+, 100â1000 m/z, Peaks: 1. MDPV, 2. Butylone, 3. Ethylone, 4. Mephedrone, 5. Methylone, 6. Methedrone.
Conclusions
HILIC chromatography involves the partition of polar analytes from a relatively nonpolar mobile phase into a semistagnant aqueous layer adsorbed onto the surface of a polar stationary phase. In addition, ion-exchange and other polar interactions are likely to occur. For these polar bases, there are multiple mechanisms of interaction to help distinguish them during a chromatographic run. Ascentis Express HILIC columns proved to be the best choice for baseline resolution of these six potentially dangerous materials sold as bath salts. Further experimentation is planned to apply this separation to a variety of biological matrices.
References
(1) LogD(7.4) and pKa values estimated using ACD/Labs PhysChem software, version 12.
(2) Standard materials kindly provided by Cerilliant Corporation (Round Rock, Texas).
Ascentis is a registered trademark of Sigma-Aldrich Co. LLC.
Supelco/Sigma Aldrich
595 North Harrison Road, Bellefonte, PA 16823
tel. (800) 359-3041, fax (800) 359-3044
Website: www.sigma-aldrich.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)















