Hot Topics in HPLC, Part VI: Chromatographic Innovations for Cell Therapies
This is sixth in a series of articles exploring hot topics in high performance liquid chromatography (HPLC).
This is sixth in a series of articles exploring hot topics in high performance liquid chromatography (HPLC).
In the opening address of 8th International HPLC Symposium in 1984, Prof. Csaba Horvath emphasized that advances in high performance liquid chromatography (HPLC) were contributing to great gains in life sciences and would play a key role in the future (1). His vision was remarkably prescient because at that time the biopharmaceutical industry was just beginning to manufacture small protein therapeutics such as insulin and growth hormones using genetically engineered microbial expression systems. Over the last three decades, we have witnessed tremendous growth in biopharmaceutical therapeutics, extending from small proteins to large antibodies that have greater power to modulate their biological targets. Innovations from the chromatographic sciences field, such as in the form of very efficient large-scale purification or high-resolution and high-throughput analytical tools, have been instrumental to this revolution.
Today, cell therapies are at the forefront of late-line hematological cancer treatments, and they may offer promise against many other diseases as well. In immuno-oncology, biopharmaceutical companies are using different types of cells and genetic editing techniques to produce cellular “living drugs” that are designed to enhance the immune system, such as chimeric antigen receptor T (CAR-T) cells designed to eradicate specific cancers. The development of these new products has been spurred by advances in multiple scientific and biomedical disciplines. The complexity and challenges of developing, manufacturing, and controlling these cell therapies offer today’s chromatographic scientists new opportunities to join the journey of discovery and innovation needed in the development and commercialization of this new drug modality.
Chromatographic Innovations for Cell Separation
The manufacture of CAR-T drug products involves, in some cases, selection of T cells from the patient´s complex starting apheresis materials, activation and transduction with vectors for genetically programming those cells to recognize and attack the tumor cells, followed by cell expansion, formulation, and cryopreservation before re-infusion into the same patient (2). To enable initial selection of the desired cells as biologically optimal starting cells, affinity chromatographic separation methods are being developed (Figure 1) (3). These chromatography technologies offer the possibility to achieve high throughput enrichment of the desired cells, and seamless integration of the upstream and downstream cell manufacturing processes with a high degree of automation.
Figure 1: Schematic overview of an automated cell-affinity chromatographic selection workflow. Target cells with specific markers are selected from heterogenous blood products by cell-affinity chromatography. Cells are bound reversibly by interaction between multimerized low-affinity Fab-fragments off the column matrix. Residuals (D-biotin and Fab-fragments) are removed from target cells by single passage over an absorber column. Microscopic images illustrate specific properties of selection and removal matrices, which confer resin functions: binding properties on matrix surface for selection versus inside the matrix void for removal.
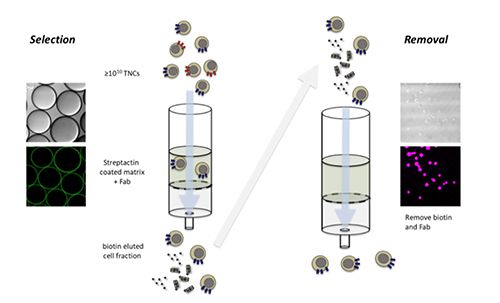
The ability to select and enrich T cells with certain phenotypic and functional attributes is important at several stages in the multistep manufacturing of complex cell products. Up-front selection of target cells reduces the complexity of patient starting materials and contributes toward a robust and consistent material for further downstream processing with fewer cell contaminants. Additionally, purification of desired individual cell populations from among the modified cells prior to final formulation, especially in the context of current sophisticated gene editing and engineering approaches, has the potential to refine the final drug product. The original high functionality of purified cells is further preserved by design of appropriate chromatographic selection technologies that allow quantitative removal of residual reagents or byproducts from cells (Figure 1).
Cell chromatographic purification technology and instrumentation in combination with next-generation bioengineering approaches provide an attractive integrated platform with many benefits. Such platforms enable a fully closed system with automation that could improve the turnaround time and reduce manufacturing costs.
Chromatographic Innovations for Analytical Methods
Analytical techniques that are typically used for product characterization and release testing of cellular therapies include flow cytometry to monitor the phenotypes of cells, and molecular analytics to confirm their genetic modification. Sample digestion and treatments that reduce sample complexity could allow more specific analysis of this new product modality by traditional chromatography. Further improved high-resolution methods may enhance the robustness and consistency of ancillary materials, such as complex culture media and various viral vectors that comprise the nascent manufacturing supply chains and are often considered critical components. For example, analytical methods using monolith columns have been developed to resolve empty from full viral capsids of adeno-associated virus (AAV)(4,5). Such a chromatographic approach may offer increased speed and improved quality control.
Gene editing is emerging as a potentially important tool to achieve desired DNA rearrangements in cellular products. Nearly 20 years ago, microscale separation for DNA such as capillary electrophoresis (CE) was instrumental in decoding the human genome. Today, CE remains a versatile genetic and molecular analysis tool with diverse applications. CE methods have been developed for profiling indels (insertions or deletions) that arise from CRISPR/Cas9-mediated genetic editing (6). The single base pair resolution of PCR amplicons by CE enables discrimination between different types of genetic modifications, and throughput can be improved in the capillary array format. Improved CE-based approaches for monitoring critical genetic modifications and other raw materials could further facilitate the development of gene-edited cellular products.
Cell expansion requires an optimal growth environment where the composition of metabolites, media components, and growth factors can significantly impact cellular phenotypes, proliferation, and differentiation. The introduction of microfluidic based CE–mass spectrometry (CE–MS) devices has allowed rapid quantification of amino acids and potentially may quantify other media components much faster than conventional HPLC-based analytics (7). The speed and throughput of new CE–MS based approaches may improve the process understanding and efficiency of cell therapy production.
Conclusions
Improving the speed and power of separations through innovation in chromatography contributes to the development of cell therapy products. In general, high resolution and high throughput preparative and analytical technologies tailored to the unique chemical and molecular properties of genetically modified cells, vectors, and their process matrices are highly desirable. Multidisciplinary, cross-functional collaborations between scientists in biopharmaceutical companies, analytical instrument companies, and academia may help to bring new and elegant separation solutions to address the challenges in the manufacture and control of cellular products. Although much has changed since 1984, the relative importance of new innovations remains constant. Along with the continuing advances in biology and engineering sciences, chromatographic innovations will help deliver the promise of new cell therapies to patients.
References:
1. C. Horvath, Opening Address for Eighth International Symposium on Column Liquid Chromatography, J. Chromatogr.316, XIII–XIV (1984).
2. C. Turtle, L.A. Hanafi, C. Berger, T. A. Gooley, S. Cherian, M. Hudecek, D. Sommermeyer et al. "CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell ALL patients." J. Clin. Investig.126,(6) 2123–2138 (2016).
3. F. Mohr , S. Przibilla, F. Leonhardt, C. Stemberger, S. Dreher, T.R. Müller, S. P. Fräßle , et al. “Efficient immunoaffinity chromatography of lymphocytes directly from whole blood.” Sci. Rep.8(1), 16731 (Nov 13, 2018).
4. C. Wang, S. Mulagapati, Z. Chen, J. Du, X. Zhao, G. Xi, L. Chen et al. "Developing an Anion Exchange Chromatography Assay for Determining Empty and Full Capsid Contents in AAV6. 2." Molec. Therapy Methods Clin, Devel.15, 257-263 (2019).
5. X. Fu, W. Chen, C. Argento, P. Clarner, V. Bhatt, R. Dickerson, G. Bou-Assaf et al. "Analytical Strategies for Quantification of Adeno-Associated Virus Empty Capsids to Support Process Development." Human Gene Therapy Methods 30, (4) 144–152 (2019).
6. L. A. Lonowski, N. Yoshiki, R. Anjum, C.E. Delay, Z. Yang, F. Niola, D. Katarzyna et al. "Genome editing using FACS enrichment of nuclease-expressing cells and indel detection by amplicon analysis." Nature Protocols12, (3) 581 (2017).
7. W. M. Gilliland and J. M.Ramsey. "Development of a Microchip CE-HPMS Platform for Cell Growth Monitoring." Anal. Chem. 90, (21) 13000–13006 (2018).
Ann L. Lee

Ann L. Lee is the Senior Vice President and Head of Cell Therapy Development and Operations, Christian Stemberger is the Senior Director of Development, Automated Cell Processing Technologies, and Taylor Zhang is the Director of Analytical Development, Residuals and Ancillary Materials, all in Global Product Development and Supply at Bristol Myers Squibb. Lee and Zhang work in Seattle, Washington, and Stemberger works in Munich, Germany. Direct correspondence to Ann.Lee@junotherapeutics.com
Best of the Week: Food Analysis, Chemical Migration in Plastic Bottles, STEM Researcher of the Year
December 20th 2024Top articles published this week include the launch of our “From Lab to Table” content series, a Q&A interview about using liquid chromatography–high-resolution mass spectrometry (LC–HRMS) to assess chemical hazards in plastic bottles, and a piece recognizing Brett Paull for being named Tasmanian STEM Researcher of the Year.
Using LC-MS/MS to Measure Testosterone in Dried Blood Spots
December 19th 2024Testosterone measurements are typically performed using serum or plasma, but this presents several logistical challenges, especially for sample collection, storage, and transport. In a recently published article, Yehudah Gruenstein of the University of Miami explored key insights gained from dried blood spot assay validation for testosterone measurement.
Determination of Pharmaceuticals by Capillary HPLC-MS/MS (Dec 2024)
December 19th 2024This application note demonstrates the use of a compact portable capillary liquid chromatograph, the Axcend Focus LC, coupled to an Agilent Ultivo triple quadrupole mass spectrometer for quantitative analysis of pharmaceutical drugs in model aqueous samples.