Hot Topics in HPLC, Part V: Exciting New Developments in Two-Dimensional Liquid Chromatography
This is fifth in a series of articles exploring hot topics in high performance liquid chromatography (HPLC).
This is fifth in a series of articles exploring hot topics in high performance liquid chromatography (HPLC).
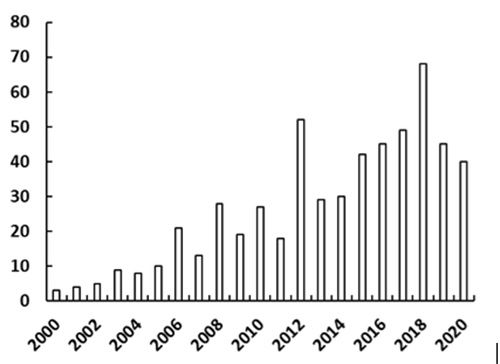
Given our research interests in multidimensional chromatography, we are naturally inclined to discuss recent developments in this area as a contribution to this “Hot Topics in LC” series. Nevertheless, the literature does show that indeed two-dimensional liquid chromatography (2D-LC) in particular is a rapidly developing area within the field of high performance liquid chromatography (HPLC). Figure 1 shows the number of publications in peer-reviewed journals in recent years that are clearly focused on applications of 2D-LC (www.multidlc.org/literature/2DLC-Applications). The current year 2020 looks to be particularly fruitful, with 40 publications already just five months into the year. For this brief piece we have elected to focus on three aspects of recent work by the 2D-LC community that we think are especially noteworthy: 1) Movement toward “generic” 2D-LC methods; 2) development of ultrahigh peak capacity methods; and 3) solutions that address the “solvent mismatch” problem in 2D-LC. Each of these is discussed in turn below.
Figure 1: Number of literature articles in recent years focused on applications of 2D-LC.
Movement Toward Generic 2D-LC Methods
When starting to develop a new 2D-LC method, one of the most common questions asked by users is, What columns should I use? For some applications the answer is straightforward. For example, if the goal is to separate tryptic peptides from one or a few proteins, this can be done very effectively by 2D-LC using either cation-exchange (CEX) or reversed-phase with a high pH (~9) in the first dimension, followed by reversed-phase at low pH (~3) in the second dimension. Excellent separations of this kind have been demonstrated in the literature, and these conditions can be adopted immediately 1). However, for other separations, and particularly when dealing with molecules that are highly similar, selectivity of the columns used is of paramount importance, and finding the “right” column can feel like finding a needle in a haystack. In conventional one-dimensional LC (1D-LC) this problem is most commonly addressed by using automated method development systems that screen a variety of mobile phases and columns in the hunt for the “needle”-the combination of conditions that will yield the best separation for the mixture at hand. In 2013 Zhang et al. implemented a column switching valve in the second dimension of a 2D-LC system that allowed automated screening of different columns to make the process of finding the best column more efficient (2). In two recent papers this year, the group of Regalado and Pickens and coworkers has taken this approach further by implementing column selection valves in the first and second dimensions of a 2D-LC system, along with a solvent selection valve in the first dimension that allows screening not only column chemistries, but also combinations of stationary and mobile phases (3,4). This group has applied this approach to both small-molecule separation challenges (3) and protein separation challenges (4). This is exciting work that should capture the imagination of anyone interested in a “set it and forget it” approach to method development, where an array of conditions that are both generic and diverse can be used as a starting point to identify candidate combinations of mobile and stationary phases that can be further optimized using traditional tools (such as, DryLab and similar tools).
Applications for Ultrahigh Peak Capacity 2D-LC
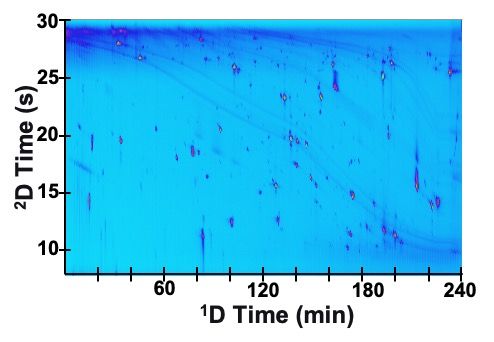
As our ability to produce 1D-LC separations with more and more resolving power continues to improve through improvements in column technology and theoretical understanding of their limitations, so too does our ability to execute 2D-LC separations with higher and higher resolving power. It was only a decade or so ago that some believed that achieving peak capacities of 10,000 or more was only practically feasible using offline 2D-LC approaches (5). Two recent publications-one from our own work, and one from the Desmet group-have shown that this barrier has fallen by the wayside. Figure 2 from shows that it is possible to achieve a peak capacity of 10,000 in an online LCxLC separation of tryptic peptides with an analysis time of four hours (6). This type of separation should be useful for those interested in separating extremely complex mixtures of peptides for subsequent identification by mass spectrometry, or for increasing the likelihood that observed peaks detected by less selective detectors (such as UV-vis) are highly pure (that is, with less co-elution). Zhu and Desmet, et al., have developed a method that is focused on identification of unknown compounds in a complex mixture of amine oligomers where chromatographic separation is of paramount importance because it makes the interpretation of mass spectra for identification much simpler (7). In this case Zhu et al. achieved a peak capacity of about 11,000, albeit in an analysis time of 11 h.
Figure 2: LCxLC separation of tryptic peptides using reversed-phase columns in both dimensions. The estimated peak capacity is 10,000 and the analysis time is 4 h. Adapted with permission from ref. (6).
Addressing Solvent Mismatch
One important topic in recent 2D-LC research is the issue of solvent mismatch (also referred to as incompatibility) between the 1D effluent and the 2D eluent. Such (vast) differences in solvent properties such as solvent strength, polarity, and viscosity, can result in in dramatic distortion of 2D peaks (8). A decade ago, this issue was, arguably, widely perceived as one of the major limitations preventing application of 2D-LC to samples that required separation modes other than reversed-phase LC . In this period, several groups were working toward the application of LC×LC to food (9–11), natural medicines (12), lipidomics (13), polymers (14,15), and surfactants (16,17) have attempted to combine organic separation modes including hydrophilic interaction chromatography (HILIC), normal-phase LC, and size-exclusion chromatography (SEC), with reversed-phase LC in the second dimension. Depending on the ordering of the separations (that is, is reversed-phase LC in the first or second dimension?) the solvent mismatch affects the feasibility of the method differently (18). While generally successful, methods had to be adjusted to prevent the occurrence of these mismatch effects, and normal-phase LC × reversed-phase LC methods were typically very long, or had to be carried out offline. More recently, however, there has been less mention in the literature of solvent mismatch as a major impediment to further adoption of 2D-LC, presumably because the recent advances discussed below have made great strides toward addressing the problem.
For the solution to this problem, several groups identified the modulation principle as the root cause. In standard LC×LC modulation, the 1D effluent is passively transferred in its entirety to the second dimension (8). This effluent comprises the analytes of interest as well as large volumes of 1D mobile-phase solvents, which in turn lead to detrimental effects on peak shape and width in the 2D column. Approaches aimed to resolve the issue have been designed to either actively remove the incompatible solvent or dilute it.
Strategies to remove the solvent include evaporation with (19) and without (12) a membrane, and adsorption-mechanisms (20) in combination with temperature (21) or active dilution of the 1D effluent (22) to facilitate retention. The critical prerequisite that determines the success of these strategies is the ability of the interface to retain all analytes during solvent removal. Unfortunately, such information has been scarcely provided for most applications utilizing this approach (23). Stationary-phase assisted modulation (SPAM) has been particularly successful for combinations where the analytes of interest could readily be retained during modulation (24,25). Although the technique is readily applied to improve detection sensitivity and analysis time (Figure 3), this retention is difficult to achieve for analytes for which the trapping sorbent is not selective, or where the mixing of the diluent flow is insufficient (26).
Approaches to dilute the solvent do not suffer from this prerequisite. A particularly robust approach that is also commercially available is active-solvent modulation (ASM) (27). In ASM, the 1D effluent is actively diluted during injection into the second dimension, and loss of analytes from the system during modulation is thus not possible. More importantly, the dilution factor can be tailored to facilitate on-column focusing on the 2D column and achieve a complementary enhanced of sensitivity. ASM has recently been applied to samples including antibodies and polymers (6,28).
Figure 3: Use of different modulation strategies over time. Data obtained from (
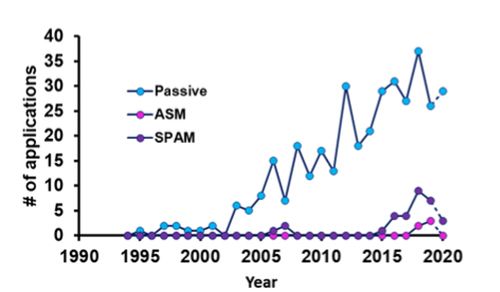
www.multidlc.org/literature/2DLC-Applications).
References
(1) K. Sandra, M. Steenbeke, I. Vandenheede, G. Vanhoenacker, and P. Sandra, The versatility of heart-cutting and comprehensive two-dimensional liquid chromatography in monoclonal antibody clone selection, J. Chromatogr. A. 1523, 283–292 (2017). https://doi.org/10.1016/j.chroma.2017.06.052.
(2) K. Zhang, Y. Li, M. Tsang, and N.P. Chetwyn, Analysis of pharmaceutical impurities using multi-heartcutting 2D LC coupled with UV-charged aerosol MS detection: Liquid Chromatography, J. Sep. Sci.36, 2986–2992 (2013). https://doi.org/10.1002/jssc.201300493.
(3) H. Wang, H.R. Lhotka, R. Bennett, M. Potapenko, C.J. Pickens, B.F. Mann, I.A.H. Ahmad, and E.L. Regalado, Introducing Online Multicolumn Two-dimensional Liquid Chromatography Screening for Facile Selection of Stationary and Mobile Phase Conditions in both Dimensions, J. Chromatogr. A. 460895, (2020). https://doi.org/10.1016/j.chroma.2020.460895.
(4) C.J. Pickens, I.A. Haidar Ahmad, A.A. Makarov, R. Bennett, B.F. Mann, and E.L. Regalado, Comprehensive online multicolumn two-dimensional liquid chromatography-diode array detection-mass spectrometry workflow as a framework for chromatographic screening and analysis of new drug substances, Anal. Bioanal. Chem. 412 2655–2663 (2020). https://doi.org/10.1007/s00216-020-02498-8.
(5) G. Guiochon, N. Marchetti, K. Mriziq, and R. Shalliker, Implementations of two-dimensional liquid chromatography, J. Chromatogr. A. 1189 109–168 (2008). https://doi.org/10.1016/j.chroma.2008.01.086.
(6) D.R. Stoll, H.R. Lhotka, D.C. Harmes, B. Madigan, J.J. Hsiao, and G.O. Staples, High resolution two-dimensional liquid chromatography coupled with mass spectrometry for robust and sensitive characterization of therapeutic antibodies at the peptide level, J. Chromatogr. B121832 (2019). https://doi.org/10.1016/j.jchromb.2019.121832.
(7) K. Zhu, M. Pursch, S. Eeltink, and G. Desmet, Maximizing two-dimensional liquid chromatography peak capacity for the separation of complex industrial samples, J. Chromatogr. A.. 1609 (2020) 460457. https://doi.org/10.1016/j.chroma.2019.460457.
(8) D.R. Stoll and P.W. Carr, Two-Dimensional Liquid Chromatography: A State of the Art Tutorial, Anal. Chem.89 519–531 (2017). https://doi.org/10.1021/acs.analchem.6b03506.
(9) P. Dugo, O. Favoino, R. Luppino, G. Dugo, and L. Mondello, Comprehensive two-dimensional normal-phase (adsorption)−reversed-phase liquid chromatography, Anal. Chem.76 2525–2530 (2004). https://doi.org/10.1021/ac0352981.
(10) P. Dugo, M. del Mar Ramirez Fernandez, A. Cotroneo, G. Dugo, L. Mondello, Optimization of a Comprehensive Two-Dimensional Normal-Phase and Reversed-Phase Liquid Chromatography System, J. Chromatogr. Sci.44 561–565 (2006). https://doi.org/10.1093/chromsci/44.9.561.
(11) K.M. Kalili and A. de Villiers, Off-line comprehensive two-dimensional hydrophilic interaction×reversed phase liquid chromatographic analysis of green tea phenolics, J. Sep. Sci.33 853–863 (2010). https://doi.org/10.1002/jssc.200900673.
(12) H. Tian, J. Xu, and Y. Guan, Comprehensive two-dimensional liquid chromatography (NPLC×RPLC) with vacuum-evaporation interface, J. Sep. Sci. 1677–1685 (2008). https://doi.org/10.1002/jssc.200700559.
(13) M. HolÄapek, M. OvÄaÄíková, M. Lísa, E. Cífková, and T. Hájek, Continuous comprehensive two-dimensional liquid chromatography–electrospray ionization mass spectrometry of complex lipidomic samples, Anal. Bioanal. Chem.407 5033–5043 (2015). https://doi.org/10.1007/s00216-015-8528-2.
(14) X. Jiang, A. van der Horst, V. Lima, and P.J. Schoenmakers, Comprehensive two-dimensional liquid chromatography for the characterization of functional acrylate polymers, J. Chromatogr. A. 1076 51–61 (2005). https://doi.org/10.1016/j.chroma.2005.03.135.
(15) J.-A. Raust, A. Brüll, C. Moire, C. Farcet, and H. Pasch, Two-dimensional chromatography of complex polymers, J. Chromatogr. A.. 1203 207–216 (2008). https://doi.org/10.1016/j.chroma.2008.07.067.
(16) Z. Wu and P.J. Marriott, One- and comprehensive two-dimensional high-performance liquid chromatography analysis of alkylphenol polyethoxylates, J. Sep. Sci.34 3322–3329 (2011). https://doi.org/10.1002/jssc.201100701.
(17) S. Abrar and B. Trathnigg, Separation of nonionic surfactants according to functionality by hydrophilic interaction chromatography and comprehensive two-dimensional liquid chromatography, J. Chromatogr. A. 1217 8222–8229 (2010). https://doi.org/10.1016/j.chroma.2010.10.118.
(18) B.W.J. Pirok, A.F.G. Gargano, and P.J. Schoenmakers, Optimizing separations in on-line comprehensive two-dimensional liquid chromatography, J. Sep. Sci.41 68–98 (2017). https://doi.org/10.1002/jssc.201700863.
(19) E. Fornells, B. Barnett, M. Bailey, E.F. Hilder, R.A. Shellie, and M.C. Breadmore, Evaporative membrane modulation for comprehensive two-dimensional liquid chromatography, Anal. Chim. Acta1000 303–309 (2018). https://doi.org/10.1016/j.aca.2017.11.053.
(20) F. Cacciola, P. Jandera, Z. Hajdu, P. Cesla, and L. Mondello, Comprehensive two-dimensional liquid chromatography with parallel gradients for separation of phenolic and flavone antioxidants, J. Chromatogr. A.. 1149 73–87 (2007). https://doi.org/10.1016/j.chroma.2007.01.119.
(21) H.C. van de Ven, A.F.G. Gargano, Sj. van der Wal, and P.J. Schoenmakers, Switching solvent and enhancing analyte concentrations in small effluent fractions using in-column focusing, J. Chromatogr. A. 1427 90–95 (2016). https://doi.org/10.1016/j.chroma.2015.11.082.
(22) A.F.G. Gargano, M. Duffin, P. Navarro, and P.J. Schoenmakers, Reducing Dilution and Analysis Time in Online Comprehensive Two-Dimensional Liquid Chromatography by Active Modulation., Anal. Chem.. 88, 1785-1793 (2016) https://doi.org/10.1021/acs.analchem.5b04051.
(23) B.W.J. Pirok, D. Stoll R., and P.J. Schoenmakers, Recent developments in two-dimensional liquid chromatography – Fundamental improvements for practical applications, Anal. Chem. 91 240–263 (2019). https://doi.org/10.1021/acs.analchem.8b04841.
(24) B.W.J. Pirok, M.J. den Uijl, G. Moro, S.V.J. Berbers, C.J.M. Croes, M.R. van Bommel, and P.J. Schoenmakers, Characterization of Dye Extracts from Historical Cultural-Heritage Objects Using State-of-the-Art Comprehensive Two-Dimensional Liquid Chromatography and Mass Spectrometry with Active Modulation and Optimized Shifting Gradients, Anal. Chem. 91 3062–3069 (2019). https://doi.org/10.1021/acs.analchem.8b05469.
(25) B.W.J. Pirok, N. Abdulhussain, T. Aalbers, B. Wouters, R.A.H. Peters, and P.J. Schoenmakers, Nanoparticle Analysis by Online Comprehensive Two-Dimensional Liquid Chromatography combining Hydrodynamic Chromatography and Size-Exclusion Chromatography with Intermediate Sample Transformation, Anal. Chem.. 89 9167–9174 (2017). https://doi.org/10.1021/acs.analchem.7b01906.
(26) M.A. Ianovska, P.P.M.F.A. Mulder, and E. Verpoorte, Development of small-volume, microfluidic chaotic mixers for future application in two-dimensional liquid chromatography, RSC Advances7 9090–9099 (2017). https://doi.org/10.1039/C6RA28626G.
(27) D.R. Stoll, K. Shoykhet, P. Petersson, and S. Buckenmaier, Active Solvent Modulation – A Valve-Based Approach to Improve Separation Compatibility in Two-Dimensional Liquid Chromatography, Anal. Chem.89 9260–9267 (2017). https://doi.org/10.1021/acs.analchem.7b02046.
(28) M. Pursch, A. Wegener, and S. Buckenmaier, Evaluation of active solvent modulation to enhance two-dimensional liquid chromatography for target analysis in polymeric matrices, J. Chromatogr. A. 1562 78–86 (2018). https://doi.org/10.1016/j.chroma.2018.05.059.
Dwight R. Stoll is a professor and co-chair of chemistry at Gustavus Adolphus College in St. Peter, MinneÂsota, and the editor of the “LC Troubleshooting” column in LCGC. He is also a member of LCGC’s editorial advisory board. Bob W. J. Pirok is an assistant professor in the Analytical Chemistry Group of the Van ‘t Hoff Institute for Molecular Sciences (HIMS) at the University of Amsterdam. Direct correspondence to LCGCedit@mmhgroup.com
Best of the Week: Food Analysis, Chemical Migration in Plastic Bottles, STEM Researcher of the Year
December 20th 2024Top articles published this week include the launch of our “From Lab to Table” content series, a Q&A interview about using liquid chromatography–high-resolution mass spectrometry (LC–HRMS) to assess chemical hazards in plastic bottles, and a piece recognizing Brett Paull for being named Tasmanian STEM Researcher of the Year.
Using LC-MS/MS to Measure Testosterone in Dried Blood Spots
December 19th 2024Testosterone measurements are typically performed using serum or plasma, but this presents several logistical challenges, especially for sample collection, storage, and transport. In a recently published article, Yehudah Gruenstein of the University of Miami explored key insights gained from dried blood spot assay validation for testosterone measurement.
Determination of Pharmaceuticals by Capillary HPLC-MS/MS (Dec 2024)
December 19th 2024This application note demonstrates the use of a compact portable capillary liquid chromatograph, the Axcend Focus LC, coupled to an Agilent Ultivo triple quadrupole mass spectrometer for quantitative analysis of pharmaceutical drugs in model aqueous samples.