Hot Topics in HPLC, Part IV: The Unique Design, Operation, and Performance Demands of Portable and “Green” LC Instrumentation
Interest in reducing the size of liquid chromatography (LC) instrumentation is currently growing for a number of reasons, each depending on a specific challenge or opportunity. This is fourth in a series of articles exploring hot topics in high performance liquid chromatography (HPLC).This is fourth in a series of articles exploring hot topics in high performance liquid chromatography (HPLC).
This is fourth in a series of articles exploring hot topics in high performance liquid chromatography (HPLC).
Interest in reducing the size of liquid chromatography (LC) instrumentation is currently growing for a number of reasons, each depending on a specific challenge or opportunity. For example, chemical reaction monitoring for optimizing the reaction conditions during synthesis of new drugs in the pharmaceutical industry or new organic chemicals in university research laboratories would benefit from a small LC instrument that could be easily placed in a hood and very close to the reaction vessel. Furthermore, small-diameter columns and low mobile-phase flow rates, which are typical for small LC systems, would further benefit chemical reaction monitoring because only very small samples would need to be withdrawn from the reaction vessel during repetitive analysis, reducing the possibility of any change in conditions that could occur by reducing the volume of reactants during the reaction period.
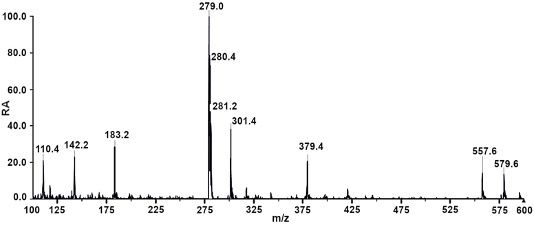
Interfacing an LC system to another instrument, such as an autosampler or a mass spectrometer, is another example application that would greatly benefit from using a small LC instrument, because the LC instrument could be placed very close to the ancillary instrumentation. In the case of LC with mass spectrometry detection (LC–MS), small-diameter columns and low mobile-phase flow rates facilitate the interfacing because the liquid flow exiting the LC column and entering the MS instrument are much better matched for optimum performance without the need for flow splitting. In Figure 1, the LC column eluent first passed through the on-column UV-absorption detector and continued to the MS.
Figure 1: UV-absorption chromatogram (top) and mass spectrum (bottom, sulfamethazine) obtained using a portable LC instrument (Axcend) coupled to a portable MS detector (Microsaic). Sample: 100 ppm sulfamethazine, carbamazepinen, ketoprofen, flavone, and amcinonide. LC conditions: 10 cm x 150 μm i.d. fused silica capillary packed with CoAnn 1.7 μm C18, 40 nL injection, 2 μL/min from 5% to 95% acetonitrile in water in 30 min, 275 nm on-column UV-absorption detection. MS conditions: 2.5 L/min nitrogen flow with 0.85 kV ESI tip voltage, positive ion mode with a full scan range from 125 to 510 m/z.
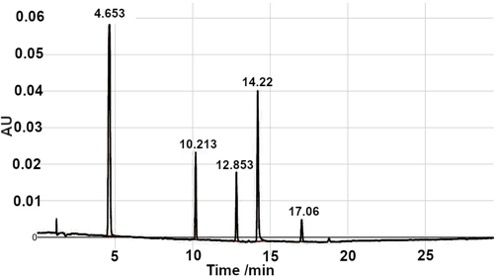
Figure 1: UV-absorption chromatogram
Conventional LC instruments that use 1.0 to 4.6 mm i.d. columns require relatively large solvent reservoirs to support the approximately 0.1 to 2 mL/min LC mobile-phase flow rates. The use of such large solvent reservoirs is cumbersome when moving an LC instrument to different locations (such as when transporting to the laboratory or other analysis site) and in terms of other aspects of solvent handling (such as storing solvents, safely disposing waste, and minimizing human exposure during those steps). By enabling significantly reduced consumption of valuable chemical resources and reduced disposal of waste, small LC systems can advance the growing trend to move to “greener” analytical techniques.
From an economic viewpoint, a small LC instrument would greatly reduce the cost of solvent use (for both purchase and disposal). Comparing solvent-use costs for a 4.6 mm i.d. column and a 0.15 mm i.d. column, for which the latter would consume approximately a thousand times less solvent, the estimated solvent-use costs would be approximately $7000 and $7 for one year of operation and 36 runs per day, respectively, which differ by approximately a thousand times, as expected. For an analytical laboratory that operates 100 LC instruments, the comparative costs would become $700,000/year versus $700/year, a major cost in savings for the small-diameter column. For comparison, the costs for 2.1 mm and 1.0 mm i.d. columns would be $140,000 and $32,000/year, respectively, which are still significantly more than $700/year.
For the reasons described above, we have been involved in the development of a small, portable LC system that was recently commercialized. This effort began in 2011 in the research laboratories at Brigham Young University, in Provo, Utah, in collaboration with Stan Stearns and engineers at VICI Valco, in Houston, Texas, to develop an ultrahigh-pressure nanoflow pumping system (1–6). The technology was subsequently licensed to Axcend LLC for product development and commercialization.
The design criteria for this LC instrument included small size, light weight, low mobile-phase consumption, low power usage, high chromatographic performance, easy operation, and good robustness. The resulting system is a hand-portable LC that has a small footprint (approximately 30 x 20 x 20 cm), weighs approximately 8 kg (including a rechargeable Li-Fe-PO4 battery), produces consistent 0.5 to 10 μL/min flow rates at pressures up to 689 bar (10,000 psi), utilizes on-column UV-absorption detection with light-emitting diode (LED) light sources, and is very easy to operate. The system has a cartridge that houses the capillary columns and detectors. Several column-detector configurations can be selected from various length columns (typically, 5, 10, and 25 cm, all 150 μm internal diameter) and one or two UV-absorbance detectors, including simply one column followed by one detector, or one column followed by two tandem detectors of different wavelengths, or a combination of two columns and two detectors where one column packed with one stationary phase is followed by a detector of a specific wavelength, and a second column packed with a different stationary phase is followed by a second detector of a different wavelength, all in series. The latter configuration can provide two complementary retention times and complementary UV absorbance values that can be used for near positive target compound identification. Alternatively, the end of the column can be extended out of the back of the cartridge for direct interfacing to a variable wavelength UV-absorbance detector or to a mass spectrometer for identification of unknown compounds.
One challenge in using very small i.d. columns is preventing tiny particles in the sample or mobile phase from plugging the extremely small i.d. sampling valve opening or the transfer line tubing that connects the injection valve to the column. For this reason, more care is required than for conventional LC systems to filter samples and mobile phases (for example, using a 0.2 micrometer pore-diameter filter) before they are introduced into the LC system. Furthermore, because UV-absorption detection is accomplished on-column immediately after the stationary phase packing in a 150 μm i.d. PTFE-coated capillary column, it is extremely important to eliminate as much detector noise as possible, which can originate from a variety of sources. The laboratory prototype nano- to micro- flow LC system gave a UV-absorption detection limit of 25 nM (7.6 ppb) for sodium anthraquinone-2-sulfonate when the detector was placed a short distance away from the pumping system and electronics, and was well shielded. Because the detector is placed inside a cartridge and near the electronic board stack in the commercial system, it has been more challenging to reduce the background noise to the same level as for the prototype detector. Therefore, the detection limits are higher, which currently restricts the use of this system for some applications that require UV-absorption detection of extremely low concentrations. We are currently working on identifying and minimizing these elusive sources of noise and increasing the detection path length to improve the detection limits.
References
- S. Sharma, A. Plistil, R.S. Simpson, K. Liu, P.B. Farnsworth, S.D. Stearns, and M.L. Lee, “Instrumentation for Hand-Portable Liquid Chromatography,” J. Chromatogr. A1327, 80–89 (2014).
- P. Aggarwal, K. Liu, S. Sharma, J.S. Lawson, H.D. Tolley, and M.L. Lee, “Flow Rate Dependent Extra-column Variance from Injection in Capillary Liquid Chromatography,” J. Chromatogr. A 1380, 38–44 (2015).
- S. Sharma, H.D. Tolley, P.B. Farnsworth, and M.L. Lee, “LED-based UV Absorption Detector with Low Detection Limits for Capillary Liquid Chromatography,” Anal. Chem.87, 1381–1386 (2015).
- S. Sharma, A. Plistil, H.E. Barnett, H.D. Tolley, P.B. Farnsworth, S.D. Stearns, and M.L. Lee, “Hand-portable Gradient Capillary Liquid Chromatography Pumping System,” Anal. Chem.87, 10457–10461 (2015).
- X. Zhao, X. Xie, S. Sharma, L.T. Tolley, A. Plistil, H.E. Barnett, M.P. Brisbin, A.C. Swensen, J.C. Price, P.B. Farnsworth, H.D. Tolley, S.D. Stearns, and M.L. Lee, “Compact Ultrahigh-Pressure Nanoflow Capillary Liquid Chromatograph,” Anal. Chem. 89, 807–812 (2017).
- X. Xie, L.T. Tolley, T.X. Truong H.D. Tolley, P.B. Farnsworth, and M.L. Lee, “Dual-wavelength LED-based UV Absorption Detector for Nano-flow Capillary Liquid Chromatography,” J. Chromatogr. A1523, 242–247 (2017).
Milton L. Lee

Milton L. Lee is the Chief Science Officer at Axcend and the Emeritus H. Tracy Hall Professor of Chemistry at Brigham Young University.
Xiaofeng Xie and Elisabeth P. Gates are research scientists, Paul A. Peaden is an applications scientist, and W.Raymond West is vice-president of customer success, all with Axcend Corporation in Provo, Utah. Leena M. Patil is a post-doctoral researcher in the Department of Chemistry and Biochemistry at Brigham Young University in Provo, Utah.
Best of the Week: Food Analysis, Chemical Migration in Plastic Bottles, STEM Researcher of the Year
December 20th 2024Top articles published this week include the launch of our “From Lab to Table” content series, a Q&A interview about using liquid chromatography–high-resolution mass spectrometry (LC–HRMS) to assess chemical hazards in plastic bottles, and a piece recognizing Brett Paull for being named Tasmanian STEM Researcher of the Year.
Using LC-MS/MS to Measure Testosterone in Dried Blood Spots
December 19th 2024Testosterone measurements are typically performed using serum or plasma, but this presents several logistical challenges, especially for sample collection, storage, and transport. In a recently published article, Yehudah Gruenstein of the University of Miami explored key insights gained from dried blood spot assay validation for testosterone measurement.
Determination of Pharmaceuticals by Capillary HPLC-MS/MS (Dec 2024)
December 19th 2024This application note demonstrates the use of a compact portable capillary liquid chromatograph, the Axcend Focus LC, coupled to an Agilent Ultivo triple quadrupole mass spectrometer for quantitative analysis of pharmaceutical drugs in model aqueous samples.