Heparin Characterization
Heparin is well-known as an anti-coagulant, antithrombotic drug. Chemically, it is a linear polysaccharide that is derived from animal tissues. For some time it has been known that heparin is not a homogeneous substance; rather, it is a heterogeneous mixture of molecules ranging in molar mass from less than 5,000 to more than 30,000 Daltons. Heparin can be chemically or enzymatically depolymerized to obtain low molecular weight (LMW) heparin products, which exhibit an improved pharmacological profile.
Heparin is well-known as an anti-coagulant, antithrombotic drug. Chemically, it is a linear polysaccharide that is derived from animal tissues. For some time it has been known that heparin is not a homogeneous substance; rather, it is a heterogeneous mixture of molecules ranging in molar mass from less than 5,000 to more than 30,000 Daltons. Heparin can be chemically or enzymatically depolymerized to obtain low molecular weight (LMW) heparin products, which exhibit an improved pharmacological profile.
The U.S. Food and Drug Administration (FDA) has scrutinized LMW-heparin products closely due to their inherent variability and polydispersity. Previously, these materials had to be characterized by relative techniques involving viscometry or standard elution-based chromatographic methods. Fortunately, the advent of multi-angle laser light scattering combined with SEC means that authentic calibration standards derived from heparin are no longer necessary. LMW-heparins may now be characterized absolutely–without regard for the molecules' elution time or conformation in solution.
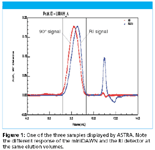
Figure 1
For this application note, Scientific Protein Laboratories (Waunakee, WI), a subsidiary of American Home Products Corporation, analyzed three commercial LMW heparin products. They employed a Waters 510 pump and 401 refractometer, a Shodex OHpak SB-803HQ analytical column (8.0 × 300 mm) with an SB-G guard column, and a miniDAWN, triple angle light scattering instrument. The mobile phase was 0.1 M ammonium acetate, and the specific refractive index increment, dn/dc was measured off-line in an Optilab operating at the same wavelength as the miniDAWN. Details of this study were published in Analytical Biochemistry 245, 231–241 (1997).
The results show unequivocally the power of the miniDAWN to resolve the three LMW-heparin products, each of which was manufactured by a different depolymerization process. Wyatt Technology's ASTRA software indicates that clear differences exits in these products' differential molar mass distributions (Figure 2).
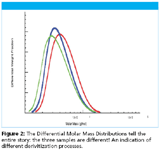
Figure 2
We sincerely thank James Knobloch and Dr. Patrick Shaklee, Scientific Protein Labs, for sharing these data with us and permitting us to publish their results.
DAWN®, miniDAWN®, ASTRA®, Optilab®, and the Wyatt Technology logo are registered trademarks of Wyatt Technology Corporation.

Wyatt Technology Corporation
6300 Hollister Avenue, Santa Barbara, CA 93117
tel.(805)681-9009, fax (805)681-0123
Website: www.wyatt.com

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis















