A Universal Method for Basic cIEF
Achieve highly reproducible cIEF separations of basic mAbs using the basic pH gradient cIEF separation method on the PA 800 Protein Characterization System.
Achieve highly reproducible cIEF separations of basic mAbs using the basic pH gradient cIEF separation method on the PA 800 Protein Characterization System.
The use of cIEF for the characterization of therapeutic monoclonal antibodies (mAbs) has been increasingly adopted in recent years. The pl determination adds a critical dimension in establishing identity, purity, post-translational modifications, and stability of therapeutic mAb preparations. Most mAbs have charge isoforms with a pl in the basic range of the pH gradient (7–10). However, cIEF in this region presents a challenge due to presence of "poor" carrier ampholytes in the basic pH region and decay of the gradient by isotachophoresis. This application note describes a robust, high resolution cIEF method that incorporates anodic and cathodic stabilizers.
Experimental Conditions
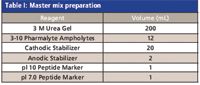
Table I: Master mix preparation
A master separation mix was prepared and 10 μL of 5 mg/mL mAb was added to 240 μL of the master mix.
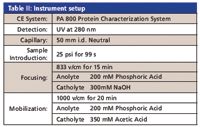
Table II: Instrument setup
Results
Electropherograms of three basic mAbs were separated using the same cIEF conditions, resulting in highly unique peak profiles and detection times (Figure 1). The unique peak profile for different mAbs shows that a single method using a broad pH 3 to 10 ampholyte mixture is capable of resolving differences in pl up to a few hundredths of 1 pH unit across the basic range of the gradient.

Figure 1
Conclusions
A single separation method can be employed to perform cIEF analysis of multiple mAbs across a broad pH range. Workflow is greatly simplified by the use of a platform method, reducing the chances for operator error and increasing overall efficiency in method development. The resulting reduction or elimination of method optimization also allows for easier scale up of cIEF analysis in routine use environments. Statistical analysis of pl and isoform group percent composition confirms that highly reproducible cIEF separations of mAbs can be achieved even in the previously problematic basic region of the pH gradient.

Beckman Coulter
4300 N. Harbor Blvd., Fullerton, CA 92853
tel. (800)742-2345, (800)643-4366
Website: www.CELeader.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














