Analysis of Melamine and Related Analogs at 1 µg/g in Infant Formula by Gas Chromatography/Mass Spectrometry
The Application Notebook
The recent establishment of a 1 μg/g safety threshold for melamine in infant foods has led to an immediate need for more sensitive methods. Here we established GC–MS conditions for highly reproducible analyses and evaluated the effectiveness of both solvent-based and matrix-matched standards. Using this method, melamine and cyanuric acid were reliably detected at and below 1 μg/g in infant formula.
The recent establishment of a 1 μg/g safety threshold for melamine in infant foods has led to an immediate need for more sensitive methods. Here we established GC–MS conditions for highly reproducible analyses and evaluated the effectiveness of both solvent-based and matrix-matched standards. Using this method, melamine and cyanuric acid were reliably detected at and below 1 μg/g in infant formula.
Recent reports linking the presence of melamine in pet food and infant formula to illnesses and deaths have led to the recall of a wide variety of tainted food products and to calls for stricter product testing. Melamine is not considered toxic alone at low doses; however, the observed toxicity has been attributed to melamine exposure in the presence of cyanuric acid. In combination, these compounds form insoluble crystals in the kidneys, causing illness and eventual renal failure. Melamine is not a legal food additive; it is a nitrogen-rich industrial compound used for plastics, flame-resistant products, and some cleaning agents. However, melamine and related byproducts are sometimes added illegally to food products in order to falsely represent the amount of protein present, since protein level in many products is determined using nonspecific assays for nitrogen content (Figure 1).
Figure 1: Melamine and related compounds are rich in nitrogen and have been used to misrepresent protein levels in some food products.
In response to the increasing need for more rigorous melamine and cyanuric acid testing, the US Food and Drug Administration (FDA) has set three different commodity-based minimum reporting levels (MRLs): 10μg/g for pet food, 2.5μg/g for human food, and 1μg/g for infant formula. These limits place stringent demands on the analyst to demonstrate adequate instrument sensitivity. The following work was performed to establish conditions for melamine analysis down to 1μg/g in infant formula and is based on the FDA method, GC-MS Screen for the Presence of Melamine, Ammeline, Ammelide, and Cyanuric Acid (1). Please refer to the FDA method for overall experimental design and semi-quantitative calculations.
Experimental Conditions
Sample Preparation
All solutions were made using a 1,000μg/mL (each compound) mixed stock solution of melamine, cyanuric acid, ammeline, and ammelide. Working standard solutions were then prepared at 1μg/mL, 10μg/mL, and 100μg/mL in extraction solvent (10/40/50 diethylamine/water/acetonitrile). The 10μg/mL working solution was used to fortify control infant formula at 0.5μg/g, 1μg/g, and 5μg/g (dry formula was prepared according to label instructions prior to fortification). These matrix spike levels were chosen to demonstrate method performance at the MRL for infant formula (1μg/g) and the MRL for adult human food commodities (2.5μg/g). High and low standards were also prepared from the working solutions. Standards were prepared in solvent alone and also in extracted matrix at concentrations equivalent to sample fortification levels (0.0125μg/mL, 0.0249μg/mL, and 0.123μg/mL) in order to evaluate possible matrix effects and to determine which technique would yield the most reliable GC–MS data.
The extraction procedure was performed as follows. Multiple 0.5g matrix samples were prepared in 50mL centrifuge tubes and fortified if necessary (matrix control blanks were not spiked). After fortification, 20mL of extraction solvent (10/40/50 diethylamine/water/acetonitrile) was added to each tube, and the samples were then sonicated for 30 min. Following sonication, the samples were centrifuged for 10 min to pellet particulate matter. The supernatant was then filtered through a 0.45μm nylon filter to remove any remaining particles. 200μL aliquots of the resulting extracts were transferred to autosampler vials for derivatization.
Derivatization
Aliquots of standards (50μL) and sample extracts (200μL) were evaporated to dryness at 70°C with nitrogen gas. Derivatization was performed as follows: 200μL pyridine, 200μL BSTFA with 1% TMCS, and 100μL of benzoguanamine (internal standard) were added to each vial. Vials were shaken or vortexed for 10 min and then incubated at 70°C for 45 min. Derivatization was also tested using 50μL of BSTFA with 1% TMCS in attempt to minimize instrument contamination from excess derivatization material (2). Samples processed with the reduced amount of reagent were not completely derivatized; therefore, the FDA method for derivatization using 200μL of reagent was followed.
Analysis
Derivatized samples and standards were analyzed on a Shimadzu QP-2010 Plus gas chromatograph mass spectrometer (GC–MS) equipped with an AOC 20i+s auto injector and sampler. An Rxi®-5Sil MS (30m × 0.25mm ID × 0.25μm) column with a 5m Integra-Guard™ integrated guard column was used for the analysis. The integrated guard column was chosen since it protects the analytical column from matrix contamination, thus extending column lifetime, yet does not leak, as separately connected guard columns with press-fit connectors can.
A splitless liner packed with wool was used to help vaporize the compounds and also to further protect the column by trapping any nonvolatile compounds. GC parameters are as follows:
Column: Rxi®-5Sil MS, 30m, 0.25mm ID,
0.25μm, w/ 5m Integra-Guard™
integrated guard (cat.# 13623-124)
Inj.: 1.0 μL splitless (hold 1 min.), 3.5 mm splitless
inlet liner
Inj. temp.: 280°C
Carrier gas: helium, constant flow
Flow rate: 1 mL/min.
Oven temp.: 75°C to 320°C @ 15°C/min.
(hold 4 min.)
Det.: MS, electrospray
The mass spectrometer was operated in SIM acquisition mode with multiple selected ions for each analyte of interest (Table I). The transfer line temperature was set at 290°C and the ion source temperature at 190°C. The filament delay was set at 8.1 min (to extend filament lifetime) and the dwell time for each ion was 0.15 s, giving a total run time of 18.67 min.
Calculations were performed using relative response factors as described in the FDA method. Samples were quantified using both solvent-based and matrix-matched quantification standards, in order to assess matrix effect and determine which procedure was more effective for infant formula.
Results
This method successfully detected melamine and cyanuric acid to the MRLs required for routine analysis of infant formula and human foods. Highly reproducible chromatographic separation was achieved (Figure 2) and was critical for compound identification, since several quantitation ions were also found in other peaks. Matrix interference was a significant issue for the analysis of ammelide and ammeline at lower concentrations.

Figure 2
Quantitation Ions and Importance of Retention Data
The quantitation ions used were chosen because they showed the highest signal for each analyte. However, several of these ions (including m/z 344 and 345) were shared among analytes and were also observed for many of the matrix peaks in both samples and derivatizing reagent blanks. This resulted in heavy reliance on chromatographic retention time data for peak identification (in conjunction with confirmation of the ion ratios listed in Table I). The FDA method requires retention times to be within 0.05 min for compound identification. This was easily achieved using the Rxi®-5Sil MS column, which produced highly reproducible results, even after the approximately 150 injections made during testing (Table II).

Table I: MS conditions (SIM mode)
Recovery of Target Compounds
Recovery data can be determined using solvent-based standards (no matrix) or matrix-matched standards (prepared in matrix extract and then derivatized). Since some matrices provide an analyte protecting effect that results in an increased response compared to an identical analysis with solvent-based standards, we evaluated both methods here (3, 4, 5). Recovery values using solvent-based standards varied from 50 to 250%, compared to the 81 to 143% recoveries shown in Table III, which were determined using matrix-matched standards. Though this method is not intended for true quantitation, recoveries based on matrix-matched standards indicate that determining melamine and cyanuric acid at different minimum reporting levels is possible, even below the 1 μg/g MRL required for infant formula.
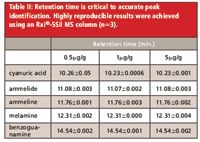
Table II: Retention time is critical to accurate peak identification. Highly reproducible results were achieved using an Rxi®-5Sil MS column (n=3).
Matrix Interference
As shown in Table III, ammelide and ammeline could not be integrated reliably below the MRL, due to partial coelution with a matrix compound that had isobaric interferences. The same effect was observed for ammeline at 1μg/g. Accurate integration was not possible since the ion ratios of the target analyte did not align with that expected based on known spectra or spectra of standards determined on the same system. However, the presence of the analytes could be confirmed by exact retention time matching of extracted ion chromatograms and manual spectral interpretation. While this method was optimized for melamine and cyanuric acid, the matrix interference seen for the other compounds illustrates the importance of evaluating and minimizing matrix carryover, especially when analyzing a new matrix. In this work, derivatizing reagent blanks were analyzed between each sample to ensure there was no carryover to subsequent injections (Figure 3).
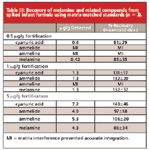
Table III: Recovery of melamine and related compounds from spiked infant formula using matrix-matched standards (n = 3).
Conclusions
Analysis of melamine and related compounds in infant formula is challenging since it has the lowest MRL of all commodities, and also because it is rich in sugars, which derivatize and chromatograph easily, thus increasing the potential for significant interferences. While this method successfully determined melamine and cyanuric acid to the MRLs required for routine analysis, coelutions complicated the analysis of ammelide and ammeline. Reliable retention time identification was critical for compound identification and the Rxi®-5Sil MS column used here detected target analytes reproducibly using the FDA method and thus is recommended for GC–MS analysis of melamine. In addition, this column includes an Integra-Guard™ integrated guard column, which extends analytical column lifetime without the risk of leaks associated with manually connected guards.

Figure 3
References
(1) US Food and Drug Administration, October 2008, GC-MS Screen for the Presence of Melamine, Ammeline, Ammelide, and Cyanuric Acid, Laboratory Information Bulletin No. 4423, http://www.cfsan.fda.gov/~frf/lib4423.html
(2) M. Long and J. Kowalski, Advantage 2008.01, 14–15 (2008).
(3) C.F. Poole, J. Chromatogr. A 1158, 241–250 (2007).
(4) T. Čajka, K. Maštovská, S.J. Lehotay and J.ajšlová, J. Sep. Sci. 28, 1048–1060 (2005).
(5) K. Maštovská, S.J. Lehotay and M. Anastassiades, Anal. Chem.77, 8129–8137 (2005).

Restek Corporation
110 Benner Circle, Bellefonte, PA 16823
tel. (800)356-1688, fax (814)353-1309
Website: www.restek.com
















2 Commerce Drive
Cranbury, NJ 08512

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)