Practical Aspects of Solvent Extraction
The Application Notebook
Columnist Ron Majors discusses some of the practical considerations in the successful application of the popular yet age-old technique of solvent extraction (also known as liquid–liquid extraction, or LLE). After a brief review of the basics, guidelines on the selection of the appropriate extraction solvents and how to use acid–base equilibria to ensure efficient extractions of ionic and ionizable compounds are provided. Problems in LLE and the solutions to these problems are highlighted. A newer technique called dispersive liquid–liquid microextraction is introduced.
Recently, I had the opportunity to participate in Colacro XII, a chromatography symposium held in a Latin America country every two years. This year's meeting was held in Florianopolous, Brazil, October 27-30. During the symposium, I perused the large number of applications posters for the sample preparation techniques being used. I was surprised to find many of the investigations of such diverse matrices as natural products, fruits and vegetables, petroleum products, and body fluids used solvent extraction (liquid–liquid extraction, or LLE) as the initial (or the only) sample preparation technique. So, I thought that I would devote an installment of "Sample Prep Perspectives" to this well used technique. In our last comprehensive sample preparation survey, nearly 40% of the respondents reported on the use of LLE as at least one of their routine sample preparation procedures (1).

Ronald E. Majors
Earlier, I wrote on the basics of LLE providing some of the theory (which will not be repeated here), examples of classical LLE including continuous and countercurrent chromatography, and some of newer techniques of the time (2). More recently, I covered approaches for miniaturization of classical LLE (3). In this installment, I will provide some practical hints to those who are considering the use or already are using LLE in hopes of improving your methodology.

Quick Review of LLE
LLE is performed using two immiscible liquids and soluble samples. LLE is useful for separating analytes from interferences by partitioning the sample between these two immiscible liquids or phases. Usually, one phase in LLE will be aqueous (often the denser or heavier phase) and the second phase is an organic solvent (usually the lighter phase). The more hydrophilic compounds prefer the polar aqueous phase, while more hydrophobic compounds will be found mainly in the organic solvent. Analytes extracted into the organic phase are recovered easily by evaporation of the solvent, while analytes extracted into the aqueous phase often can be injected directly onto a reversed-phase high performance liquid chromatography (HPLC) column. Obviously, when the analyte of interest in found in the aqueous phase, evaporation is a much slower process. However, the following discussion assumes that an analyte of interest is concentrated preferentially into the organic phase, but similar approaches are used when the analyte is extracted into an aqueous phase.
The generic flow diagram of Figure 1 summarizes the steps involved in a typical LLE separation. Because extraction is an equilibrium process with limited efficiency, significant amounts of the analyte can remain in both phases. Chemical equilibria involving changes in pH, ion-pairing, and complexation can be used to enhance analyte recovery and eliminate interferences.

Figure 1
In its classical and simplest form, LLE is performed conveniently in a separatory funnel where the two immiscible phases are added from the top and, after the extraction process, the heavier phase is drained out the bottom stopcock. However, LLE can be performed in other devices such as beakers, vials, centrifuge tubes, and graduated cylinders. In these cases, the liquids must be removed by some type of pipette and great care must be exercised not to disturb the interface layer. Otherwise, the extraction process may be incomplete.
Selection of the Organic Solvent
The LLE organic solvent is chosen for the following characteristics:
- A low solubility in water (<10%).
- Volatility for easy removal and concentration after extraction.
- Compatibility with the HPLC or gas chromatography (GC) detection technique to be used for analysis (for example, avoid solvents that are strongly UV-absorbing for LC and chlorinated solvents for GC using electron-capture detection).
- High purity because extracted analytes often are recovered by the evaporation of large volumes of organic solvent; impurities in the solvent can be concentrated as well.
- Polarity and hydrogen-bonding properties that enhance recovery of the analytes in the organic phase — that is, increase the value of KD, the distribution constant (1).
Several approaches can be used to increase the value of KD:
- The organic solvent can be changed.
- If the analyte is ionic or ionizable, its KD can be increased by suppressing its ionization to make it more soluble in the organic phase. The analyte also can be extracted into the organic phase by ion pairing provided that the analyte is ionized and a suitable ion-pair reagent is added to the organic phase.
–"Salting out" can be used to decrease an analyte's concentration in the aqueous phase, by addition of an inert, neutral salt (for example, sodium sulfate) to the aqueous phase.
Table I provides examples of typical extraction solvents, as well as some unsuitable (water-miscible) extraction solvents. Apart from miscibility considerations, the main selection criteria is the polarity P' of the solvent in relation to that of the analyte. Maximum KD occurs when the polarity of the extraction solvent matches that of the analyte. For example, the extraction of a polar analyte from an aqueous sample matrix would be best accomplished with a more polar (large P') organic solvent. An optimum-polarity organic solvent can be selected conveniently by blending two solvents of different polarity (for example, hexane and chloroform), and measuring KD versus the composition of the organic phase (4). A solvent mixture that gives the largest value of KD is then used for the LLE procedure. Further changes in KD can be achieved, with improvement in the separation of analytes from interferences, by varying organic-solvent selectivity. Solvents from different regions of the solvent-selectivity triangle (5) are expected to provide differences in selectivity.

Table I: Extraction solvents for LLE(10)*
The Practice of LLE
In solvent extraction, the organic species of interest sometimes can be transferred into either phase, depending upon the selected conditions. For example, consider the extraction of an organic basic analyte (a primary amine) from an aqueous solution. If the aqueous phase is buffered at least 1.5 pH units above its pKa value, the analyte will be neutral and prefer the organic phase; more polar interferences will be extracted into the aqueous phase. If the pH of the aqueous solution is lowered (<<pKa), so that the analyte is now ionized (protonated), it will be extracted into the aqueous phase, leaving less polar interferences in the organic phase. The principles of acid–base extraction as a function of pH are the same for LLE and HPLC.
If the analyte KD is unfavorable, additional extractions may be required for improved recovery. In this case, a fresh portion of immiscible solvent is added to extract additional solute and all extracts are combined. Generally, for a given volume of final extracting solvent, multiple extractions are more efficient in removing a solute quantitatively than the use of a single extraction volume. Back extraction can be used to further reduce interferences. For example, consider the previous example of a basic analyte. If the analyte is first extracted at high pH into the organic phase, polar interferences (for example, hydrophilic neutrals, ionized acids) are left behind in the aqueous phase. If a fresh portion of low-pH aqueous buffer is used for the back-extraction of the organic phase, the ionized basic compound is transferred back into the aqueous phase, leaving nonpolar interferences in the organic phase. Thus, a two-step back-extraction allows the removal of both acidic and neutral interferences, whereas a one-step extraction can eliminate one or the other of these interferences, but not both.
If KD is very small or the required quantity of sample is large, it becomes impractical to carry out multiple extractions for quantitative recovery of the analyte. Too many extractions are required, and the volume of total extract is too large. Also, if the extraction is slow, a long time might be required for equilibrium to be established. In these cases, continuous LLE can be used, where fresh solvent is recycled continually through the aqueous sample. Continuous extractors using heavier-than-water and lighter-than-water solvents have been described (6).
For more efficient LLE, a countercurrent distribution apparatus can provide a thousand or more equilibration steps (but with more time and effort). This allows the recovery of analytes having extremely small KD values; countercurrent distribution also provides a better separation of analytes from interferences. Small-scale laboratory units are available commercially. For further information on these devices, see (7).
In some cases, LLE can enhance analyte concentration in the extract fraction. Equation 1 shows the fraction of analyte extracted (E):
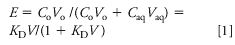
where KD is the distribution constant, Co is the concentration of the analyte in the organic phase, Caq is the concentration of the analyte in the aqueous phase, Vo is the volume of organic phase, Vaq the volume of aqueous phase, and V is the phase ratio Vo/Vaq.
Thus, by choosing a smaller volume of organic solvent, the analyte concentration can be increased by the volumetric ratio of organic to aqueous phases (assuming complete extraction into the organic phase). For example, assume 100 mL of aqueous sample, 10 mL of organic solvent, and a large KD (for example, KD > 1000). The concentration of the analyte in the organic phase will then increase by a factor of 10. For large ratios of aqueous to organic solvents, the organic solvent can be slightly soluble in the aqueous phase. This effect can reduce the volume of the recovered organic solvent and make the calculation of analyte recovery uncertain. This problem can be avoided by presaturating the aqueous solvent with organic solvent.
Microextraction
Note that when the solvent ratio V0/Vaq is small, the physical manipulation of two phases becomes more difficult. Microextraction is another form of LLE where extractions are carried out with organic–aqueous ratios of 0.001 to 0.01. In classical microextraction, analyte recovery can suffer compared to conventional LLE, but the analyte concentration in the organic phase is increased greatly and solvent usage is reduced greatly. Such extractions are carried out conveniently in a volumetric flask. In classical microextraction, the organic extraction solvent is chosen to have a density less than that of water, so that the small volume of organic solvent accumulates in the neck of the flask for easy removal. For quantitative analysis, internal standards should be used and extractions of calibration standards carried out.
A new variation of microextraction, called dispersive liquid–liquid microextraction (DLLME) has been applied successfully to a variety of analyte–matrix pairs (8). The technique is based upon a three-component solvent system. In DLLME, the container is usually a centrifuge tube and the appropriate mixture of immiscible organic extraction solvent that is heavier than water (usually a few microliters, ~8 μL of tetrachloroethylene) and a dispersive solvent (for example, a milliliter or so of acetone) is injected rapidly into an aqueous solvent (approximately 5 mL) with a syringe. The role of the dispersive solvent is to ensure miscibility between the organic phase and the aqueous phase. When the solvents are mixed rapidly, a cloudy solution is formed. This cloudy solution actually consists of fine particles (droplets) of extraction solvent, which is dispersed entirely into the aqueous phase. Because the solution is already finely dispersed, no vigorous shaking is required. Extraction time is almost instantaneous and much faster than solid-phase microextraction (SPME) and liquid-phase microextraction (LPME), which often can require 30 min or longer. Next, the entire mixture is centrifuged (1.5 min at 6000 rpm) and organic extraction solvent fine droplets are sedimented in the bottom of the centrifuge tube and are removed with a microsyringe or micropipette. The organic solution containing the analyte of interest can be injected directly or evaporated and taken up in a more appropriate solvent for the chromatographic technique. In their original work, Rezaree and colleagues (8) were able to achieve E values of approximately 600–1100 for polynuclear aromatic hydrocarbons from a water sample with excellent recoveries.
Recently, Caldas and coworkers (9) were able to show excellent extractions of pesticides (carbofuran, clomazone, and tebuconazole) in aqueous samples using DLLME along with LC with tandem mass spectrometry detection (MS-MS). For their work, they found that a 60-μL portion of extraction solvent (carbon tetrachloride) and 2 mL of dispersive solvent (acetonitrile) for a 5-mL sample of fortified water acidified with phosphoric acid at pH 2.0 gave the best results. Because carbon tetrachloride gave chromatographic problems, they evaporated the solvent to dryness and dissolved the residue in HPLC-grade methanol. The variables in DLLME method development include: choice of dispersion solvent and its volume, choice of extraction solvent and its volume, pH if necessary, and centrifuge speed. Figure 2 shows the results of their optimization studies on extraction solvent and extraction solvent volume (9). For all three pesticides, the linear range was found to be 0.001–1.0 mg/L and the limit of quantitation (LOQ) was 0.02 mg/L. Overall, the DLLME technique is simple and fast, provides good recovery, is low cost, and provides good enrichment factors.

Figure 2
Problems Experienced in LLE
Some practical problems associated with classical LLE include
- Emulsion formation
- Analytes strongly sorbed to particulates
- Analytes bound to high molecular weight compounds (for example, drugs to proteins)
- Mutual solubility of the two phases
Emulsions are a problem that can occur with certain samples (for example, fatty matrices) and certain solvent conditions. An emulsion represents a dispersion of organic liquid within the aqueous environment inside the separatory funnel and has a lack of boundary between the organic and aqueous phases. If emulsions are not "broken" with a sharp boundary between the aqueous and organic phases, analyte recovery can be affected adversely. Emulsions can be broken by
- Addition of salt to the aqueous phase
- Heating or cooling the extraction vessel
- Filtration through a glass wool plug
- Filtration through phase separation filter paper
- Addition of a small amount of different organic solvent
- Centrifugation
If particulates are present in a sample, adsorption onto the particulates can result in a low recovery of the analyte. In such cases, washing the particulates after filtration with a stronger solvent will recover the adsorbed analyte; this extract should be combined with the analyte phase from LLE. A "stronger" solvent for recovering adsorbed analyte might involve a change in pH, increase in ionic strength, or the use of more-polar organic solvents.
Compounds normally recovered quantitatively in LLE can bind to proteins when plasma samples are processed, resulting in low recovery. Protein-binding is especially troublesome when measuring drugs and drug metabolites in biological fluids. Techniques for disrupting protein binding in plasma samples include
- Addition of detergent
- Addition of organic solvent, chaotropic agents, or strong acid
- Dilution with water
- Displacement with a more strongly binding compound
"Immiscible" solvents have a small but finite mutual solubility, and the dissolved solvent can change the relative volumes of the two phases. Therefore, it is a good practice to saturate each phase with the other, so that the volume of phase containing the analyte can be known accurately, allowing an optimum determination of analyte recovery. The simplest procedure for saturation is to equilibrate the two phases in a separatory funnel without the sample, thereby saturating each phase. Aliquots of either phase can then be used for LLE.
Conclusions
The use of LLE will continue to be a mainstay sample preparation technique for years to come. The technique is simple, does not require expensive equipment, is fairly inexpensive, and provides good extraction efficiency and recovery. Many methods are published in the literature and method development is relatively simple, relying upon differences in polarity of analytes, matrices, extraction solvents and conditions. However, in its classical form, LLE is manually intensive, requires hood and bench space if many samples are to be extracted at once, and uses copious amounts of organic solvent. Problems such as emulsion formation can occur but there are techniques to minimize such occurrences. Automation of classical LLE can be quite expensive but some autosamplers and liquid-handling systems can now perform multiple extractions on milliliter amounts of extraction solvents in unattended operation. Miniaturization of the technique has been developed and now accepted (3).
Ronald E. Majors "Sample Prep Perspectives" Editor Ronald E. Majors is Senior Chemist, Columns and Supplies Division, Life Sciences Chemical Analysis, Agilent Technologies, Wilmington, DE, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com.
References
(1) R.E. Majors, LCGC 20(12), 1098–1113 (2002).
(2) R.E. Majors, LCGC 14(11), 936–949 (1996).
(3) R.E. Majors LCGC 20(2), 118–139 (2006).
(4) L.R. Snyder, Chemtech 9, 750 (1979).
(5) L.R. Snyder, Chemtech 10, 188 (1980).
(6) T.S. Ma and V. Horak, Microscale Manipulations in Chemistry (Wiley, New York, 1976).
(7) T.P. King and L.P. Craig, in Methods in Biochemical Analysis, Vol. 10, D. Glick, Ed. (Interscience Publishers, New York, 1962).
(8) M. Rezaee, Y. Assadi, M-R. M. Hosseini, E. Aghaee, F. Ahmadi, and S. Berijani, J. Chromatogr., A 1116, 1–9 (2006).
(9) S.S. Caldas, F.P. Costa, and E.G. Primel, XII Congresso Latino-Americano de Chromatografia E Technicas Relacionadas (Colacro XII), Florianopolis, Brazil, October 27–30, 2008, poster Tu-145.
(10) R.E. Majors in L.R. Snyder, J.J. Kirkland, and J. Glajch, Practical HPLC Method Development, 2nd edition (Wiley-Interscience, New York, 1997), p.115.

Determining the Effects of ‘Quantitative Marinating’ on Crayfish Meat with HS-GC-IMS
April 30th 2025A novel method called quantitative marinating (QM) was developed to reduce industrial waste during the processing of crayfish meat, with the taste, flavor, and aroma of crayfish meat processed by various techniques investigated. Headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) was used to determine volatile compounds of meat examined.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



















