Determination of Phenolic Compounds Using HPLC and Electrochemical Detection with Disposable Carbon Electrodes
The Application Notebook
Phenols are frequently present in water because of their widespread use in commercial products and because they are by-products of processes in petrochemical, pulp and paper, plastic, and glue manufacturing industries (1,2). The concentration of phenolic compounds in the waste discharges can be as high as 20 mg/L (2); however, phenol-containing pesticides and wood preservatives may cause significant health hazards even at mg/L levels (1). Consequently, it is important to monitor phenols and substituted phenols in environmental and biological samples. Liquid chromatography with electrochemical detection is one of the widely used methods due to its high selectivity and sensitivity for phenolic compounds. However, glassy carbon working electrodes, used in the electrochemical detection of phenols, often require polishing (3). This time-consuming and often poorly reproducible polishing can be avoided with disposable carbon electrodes, which offer comparable or better analytical performance (4).
Phenols are frequently present in water because of their widespread use in commercial products and because they are by-products of processes in petrochemical, pulp and paper, plastic, and glue manufacturing industries (1,2). The concentration of phenolic compounds in the waste discharges can be as high as 20 mg/L (2); however, phenol-containing pesticides and wood preservatives may cause significant health hazards even at μg/L levels (1). Consequently, it is important to monitor phenols and substituted phenols in environmental and biological samples. Liquid chromatography with electrochemical detection is one of the widely used methods due to its high selectivity and sensitivity for phenolic compounds. However, glassy carbon working electrodes, used in the electrochemical detection of phenols, often require polishing (3). This time-consuming and often poorly reproducible polishing can be avoided with disposable carbon electrodes, which offer comparable or better analytical performance (4).
Experimental
- Dionex ICS-3000 system consisting of a gradient pump with online degassing, temperature-controlled column compartment, an electrochemical detector, and an autosampler.
- Phenols were separated with an Acclaim® 120 C18, 3 μm analytical column (2.1 × 100 mm) at a flow rate of 0.20 mL/min. The injection volume was 10 μL. Analytes were detected with a disposable carbon electrode. The working electrode was held at +1.00 V vs. Ag/AgCl (3 M KCl) reference electrode.
Results
In Figure 1, the four representative phenols are separated in 7 min under the isocratic chromatographic conditions. The detection potential was optimized by varying applied potentials between +0.50 and +1.20 V in 0.10 V increments and comparing the peak areas and signal-to-noise ratios. As the applied potential increases, both the peak areas and noise increase. The maximum signal-to-noise ratio is achieved at the applied potential of 1.0 V. The optimized detection potential of 1.0 V remains the best choice for methanol and water ratios varied between 50:50 and 30:70 (CH3OH: H2O). Small adjustments of methanol concentration may be necessary for actual samples or if additional phenolic compounds are included in the standard mixture. The limits of detection are 0.32–2.56 μg/L with direct injection of a 10 μL sample or standard. Detection limits can be improved if larger sample volumes are injected or if a sample preconcentration is applied (3). The calibration curves are linear over three or four orders of magnitude depending on the analyte. The electrode-to-electrode reproducibility was evaluated using five disposable carbon electrodes. The relative standard deviation for all results with five electrodes was determined to be less than 15%.
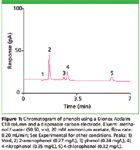
Figure 1
Conclusions
The disposable carbon electrode is a promising new tool for highly reproducible determination of low concentration of phenolic compounds in aqueous samples.
References
1. Sheikheldin, S. Y.; Cardwell, T. J.; Cattrall, R. W.; Luque de Castro, M. D.;
Kolev, S. D. Anal. Chim. Acta 2000, 419, 9–16.
2. Terashima, C.; Rao, T. N.; Sarada, B. V.; Tryk, D. A.; Fujishima, A. Anal. Chem. 2002, 74, 895–902.
3. Ruana, J.; Urbe, I.; Borrull, F. J. Chromatogr., A, 1993, 655, 217–226.
4. Cheng, J.; Jandik, P. J. Chromatogr., A 2008, 1198-1199, 148–152.
Acclaim is a registered trademark of Dionex Corporation.

Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA 94088-3603
tel. (408)737-0700, fax (408)730-9403
Website: www.dionex.com

Pick Your Poison. Isolation of Paclitaxel (Mar 2025)
March 7th 2025The diterpenoid, paclitaxel, which was identified as a potent chemotherapy agent for breast and ovarian cancer originates from the Pacific Yew tree. The isolation of paclitaxel from its major impurities is shown with the use of Hamilton’s PRP-1 (5 µm) HPLC column.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














