From Gas to Gas: Fundamentals of Static Headspace Extraction-Gas Chromatography
Headspace sampling is one of the staple sample introduction techniques for gas chromatography (GC) because it involves the injection of samples already in the vapor phase into the instrument. In this installment, we discuss the fundamentals of headspace extraction, including static versus dynamic extraction, establishing equilibrium in the vial, consequences of the partition coefficient, temperature, pressure, and transfer to the gas chromatograph. We see how, with consideration of the fundamental chemical equilibria involved, headspace extraction with GC can be one of the simplest yet most powerful and versatile chromatographic techniques.
Headspace sampling or extraction involves the collection of an aliquot of the vapor present, usually in a closed vial, above a liquid or solid sample placed within the vial. Not surprisingly, headspace sampling is among the most popular sampling or sample preparation methods for gas chromatography (GC), since, following the vaporization of sample components in the vial, the vapor is then injected or transferred to the gas chromatograph as vapor, eliminating the need for a liquid-based injection system (1,2). Most modern headspace sampling instruments still use the classical split or splitless inlet to complete sample transfer to the column.
Headspace extraction is usually performed in one of two ways. In static headspace extraction (SHE), a sample is placed in a vial, the vial is sealed (and possibly pressurized), and the sample-vial system is brought to equilibrium at a chosen temperature. Once equilibrium is established, an aliquot of the vapor within the vial is collected, either using a gas tight syringe or an automated set of valves and transferred to the gas chromatograph for analysis. SHE is most often used for routine applications where analyte concentrations in a typical liquid matrix are in the high part-per-billion (ppb) range or higher.
For lower concentration samples, dynamic headspace extraction (DHE), commonly called purge and trap, is often used. In this case, gas, usually helium or nitrogen, is continuously passed through the sample and over a sorbent trap, where vaporized analytes are sorbed and collected. Analytes in the tap are then desorbed into the gas chromatograph by flowing carrier gas through the trap and heating it. While SHE is an equilibrium technique, DHE uses the drive to equilibrium, as described in LeChatelier’s Principle, and can qualitatively remove all the analyte from a sample. This allows determination of much lower analyte concentrations. Purge and trap is often used in ultratrace analysis of contaminants in drinking water and similar applications. Going forward, this discussion will focus on SHE.
The fundamentals of SHE are discussed in detail in the classic work by Ettre and Kolb, which, while written nearly 20 years ago, is still timely (3). They provide details about the theory and fundamentals of SHE, along with instrumental details and practical hints regarding vials, septa, and operational techniques.
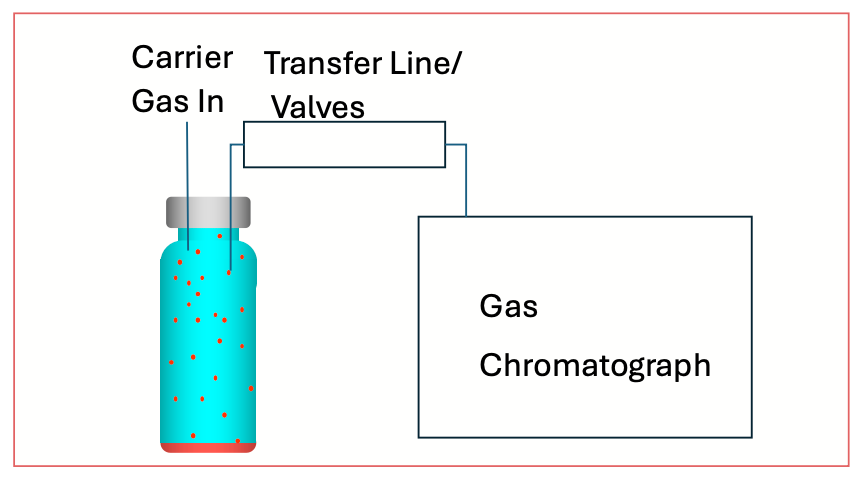
Figure 1 shows a simple diagram of a setup for SHE-based analysis. In a typical instrumental setup, once a sample is loaded into a vial, the vial is then temperature- and pressure-equilibrated using heating and carrier gas to mildly elevated temperature and pressure. Care should be taken to ensure that the temperature is high enough to facilitate vaporization of analytes, but not too much that there is either vaporization of the solvent or liquid matrix or degradation of the analytes.
FIGURE 1: Simplified diagram of an instrument for SHE. The heated and pressurized vial is directly connected to the gas chromatograph using a transfer line and a series of valves to control the injected sample volume.
New users can get started with SHE very easily. All that is needed for a simple experiment with limited temperature control are a vial and a gas tight syringe. Simply place the sample in the vial and use the gas tight syringe to collect an aliquot of the vapor in the vial headspace, then inject the collected vapor into the gas chromatograph. This method is implemented in an automated version with temperature control of both the vial and syringe on today’s rail-based autoinjectors.
Instrumental SHE devices are available from several vendors, and they generally operate on similar principles. The vial is heated and pressurized with carrier in a small oven with precise controls on the temperature and pressure. Through a series of timed valves and a transfer line, the vial is connected to a classical split or splitless inlet. The pressurized vial is opened to the inlet through the transfer line for a precisely controlled time to transfer an aliquot of the headspace to the gas chromatograph.
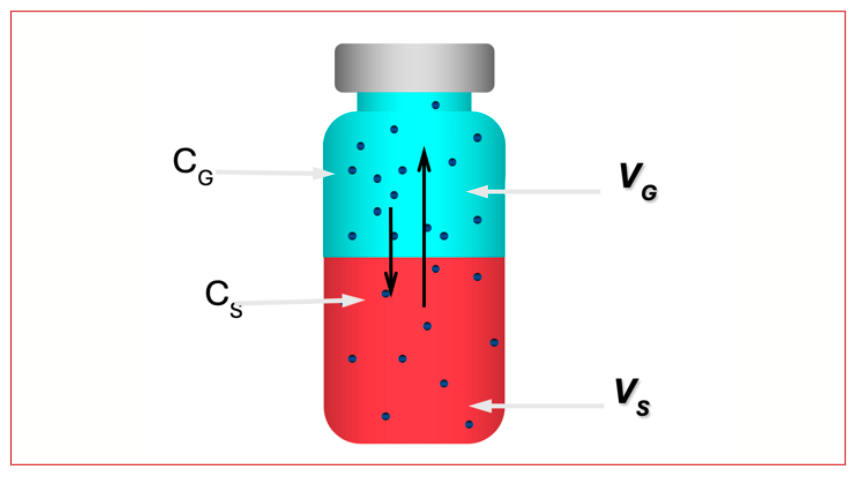
Figure 2 shows a diagram of a vial, prepared for SHE. The vial contains an aliquot of sample solution and is sealed at the top, usually using a septum and crimp or screw top. When allowed to stand long enough, the interior of the vial will come to an equilibrium condition, with masses of solvent and analyte vapor in the headspace above the solution determined by the partition coefficient of the equilibrium process.
FIGURE 2: Diagram of a sealed vial for SHE. Red represents a liquid sample; blue represents the headspace; dots represent analyte. In this vial, there are 14 dots in the headspace and 10 dots in the sample, representing a partition coefficient (K) of 10/14 or 0.7.
Equation 1 shows the simple chemical equation and equilibrium constant expression that determines the concentration of analyte in the headspace above a sample solution. Note that this is very similar to the mobile phase–stationary phase equilibrium expression seen in gas chromatography and in other phase transfer equilibria such as liquid-liquid extraction. In this equilibrium constant expression, the partition coefficient is inverted when compared to gas chromatography. In gas chromatography, the solution phase is in the numerator (product) and the vapor phase is the denominator (reactant); in this equation, the vapor phase is in the numerator and the solution phase is in the denominator.

However, for method development in SHE, Kolb and Ettre derived an expression, seen in equation 2, that includes the partition coefficient, but it is expressed in a consistent manner with GC, with the solution phase in the numerator and the vapor phase in the denominator.
Equation 2 relates the conditions within a vial for SHE and the peak area obtained at the end of the analysis. The final peak area is proportional to the initial concentration of the analyte in the sample phase divided by the sum of the partition coefficient and the phase ratio. In SHE, the phase ratio is the same as for GC, the ratio of the vapor phase to the solution phase.

We can use Equation 2 and the fundamentals of solution-vapor equilibrium to examine several method development-related situations in SHE, including the impact of partition coefficient, phase ratio (sample size), temperature, and pressure. In equation 2, a proportionality symbol is used to account for variations due to conditions in the gas chromatograph that may influence the peak area, such as inlet conditions, detector choice, and response factor.
Temperature
As we learned in introductory chemistry courses and in the fundamentals of gas chromatography, increased vial temperature moves solution-vapor equilibria, as shown in equation 1, to the vapor phase. For the partition coefficient seen in equation 2, this would make the denominator smaller, decreasing the partition coefficient (making the denominator in the fraction smaller), increasing the value of the fraction, and therefore increasing the resulting peak area. This makes intuitive sense; higher vial temperature results in more analyte in the vapor, resulting in a higher peak area and all other variables being equal.
A second consideration with temperature is solute-solvent or matrix intermolecular interactions, commonly called matrix effects. If these effects are strong, temperature may have a lower-than-expected impact on vaporization and peak area. Essentially, strong interactions between the analyte and the matrix reduce the impact of temperature on the partition coefficient, reducing the impact on the final peak area. An interesting consequence of matrix effects can occur when non-polar solutes are dissolved in polar solvents at low concentrations. In this case, matrix effects can enhance vaporization as the non-polar solute is repelled by the polar solvent.
Phase Ratio and Partition Coefficient
This leads to a discussion of the relationship of the partition coefficient and the phase ratio, seen as a sum in the denominator of equation 2. In most SHE methods, the phase ratio, volume of the vapor divided by volume of the liquid, varies between about 1–20. Consideration of the phase ratio together with the partition coefficient can be very helpful in method development. If the partition coefficient has a similar order of magnitude to the phase ratio, often seen in the situations of weak interactions or matrix effects between solute and matrix, or with volatile analytes, then the phase ratio will impact the peak area, with a larger phase ratio increasing the denominator and reducing the eventual peak area. In this situation, the phase ratio should be minimized, but this will require larger sample volume.
If the partition coefficient is much larger than the phase ratio, the case with low volatility analytes or strong matrix effects, then the phase ratio has little effect on the final peak area. Beware that a very small sample volume with a volatile solvent can result in much of the solvent evaporating if the vial is heated, affecting reproducibility. If the partition coefficient is very low, much lower than the phase ratio, the situation with highly volatile analytes, then the phase ratio has a large impact on the peak area. In this case, sample volume should be very carefully controlled, as small variations in the volume will lead to variation in the phase ratio, leading to variation in the final peak area.
Vial Pressure
Pressure in the vial has a minor influence on the partition coefficient and no influence on the phase ratio. As we learned in introductory chemistry, adding an inert gas, such as the carrier gases nitrogen or helium, does not change the equilibrium position of the reaction at constant volume. However, higher pressure in the vial may cause more of the vapor to be sampled into the transfer line in a given sampling time.
In SHE, the vial temperature, pressure, phase ratio, concentration of the analyte solution, and matrix effects all combine to determine the concentration of analyte in the vapor phase within the vial, the headspace. The analyte vapor concentration in the headspace then determines the mass of analyte, and the peak area is eventually determined in the gas chromatographic analysis. For reproducible quantitative analysis in SHE, like other extraction techniques, care should be taken to ensure that the two-phase system in the vial has reached dynamic equilibrium between the solution and vapor prior to sampling the vapor phase and injecting into the gas chromatograph. Failing to achieve equilibrium in an extraction system is the leading cause of reproducibility problems with analytical methods involving extraction, including SHE.
Conclusions
Static headspace extraction (SHE) is among the most common sampling and sample preparation techniques used in combination with gas chromatography. Conceptually, SHE is very simple: inject a sample of the vapor above a liquid or solid in a sealed vial. In practice, there are several method development considerations, based on solution or sample-vapor equilibrium, temperature, pressure and volume of the sample and vapor phases in the vial. Users can start with SHE by using a simple vial and gas tight syringe and can progress to fully automated instrumental configurations. Many autoinjectors include SHE capabilities as an option.
References
(1) Raynie, D. E. Trends in Sample Preparation Part II: Sample Considerations and Techniques. LCGC International 2024, 1 (3) 12–21. DOI: 10.56530/lcgc.int.mn3284n6
(2) Snow, N. H. Reflecting on the Current State of Sample Preparation on GC Part 2: Techniques. LCGC International 2024, 1 (5), 24–26. DOI: 10.56530/lcgc.int.sk7675y1
(3) Ettre, L. S.; Kolb, B. Static Headspace Gas Chromatography Theory and Practice, 2nd ed., John Wiley and Sons, 2006.
About the Author
Nicholas H. Snow is the Founding Endowed Professor in the Department of Chemistry and Biochemistry at Seton Hall University, and an Adjunct Professor of Medical Science. During his 30 years as a chromatographer, he has published more than 70 refereed articles and book chapters and has given more than 200 presentations and short courses. He is interested in the fundamentals and applications of separation science, especially gas chromatography, sampling, and sample preparation for chemical analysis. His research group is very active, with ongoing projects using GC, GC–MS, two-dimensional GC, and extraction methods including headspace, liquid–liquid extraction, and solid-phase microextraction. Direct correspondence to: Nicholas.Snow@shu.edu


Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.












