A Flexible SPE-LC–MS–MS Platform for the Simultaneous Quantification of Multiple Amyloid β Peptides in Cerebrospinal Fluid
Fast, flexible platforms for peptide quantification are needed, particularly for a discovery setting.
Erin E. Chambers1, Mary E. Lame2, and Diane M. Diehl1, 1Waters Corporation and 2Pfizer, Neuroscience Research Unit
Fast, flexible platforms for peptide quantification are needed, particularly for a discovery setting. This type of methodology would be especially advantageous in the case of amyloid beta (aβ) peptides. The deposition/formation of insoluble aggregates, or plaques, of aβ peptides in the brain is considered to be a critical event in the progression of Alzheimer's Disease (AD) and thus has the attention of many researchers. Historically, quantification of aβ peptides in biological fluids has relied mainly on the use of immunoassays, such as ELISA. These assays are time consuming, expensive to develop and labour intensive. They are subject to cross reactivity and an individual assay is required for each peptide. To meet the throughput requirements and constant flow of demands for new peptide methods in a discovery setting, there is a need for a highly specific yet flexible methodology based on an LC–MS-MS platform. In this work, this platform is coupled with selective sample preparation for the simultaneous quantification of multiple aβ peptides. This work focuses on methods for the 1-38, 1-40, and 1-42 aβ peptides, in support of preclinical studies.

Figure 1: Sequence, MW, and pI information for amyloid β peptides.
Experimental

Mass spectrometry
MS was performed in positive ion mode because CID of the 4+ precursor ion yielded several distinct product ions corresponding to inherently specific b sequence ions (representative spectrum shown in Figure 2).
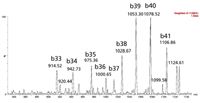
Figure 2: Representative ESI+ MS-MS spectrum for amyloid β 1-42 with fragment sequence ions labelled.
UPLC separation
Separation of the three amyloid β peptides is shown in Figure 3.
Sample preparation: SPE
SPE was performed using Oasis MCX, a mixed-mode sorbent, to enhance selectivity of the extraction. The sorbent relies on both reversed-phase and ion exchange retention mechanisms to selectively separate the aβ fraction from other high abundance polypeptides in complex CSF samples. The Oasis μElution plate (96-well format) provided significant concentration while eliminating evaporation and reconstitution, minimizing peptide losses.
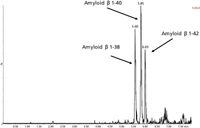
Figure 3: UPLCâMS-MS analysis of amyloid β 1-38, 1-40 and 1-42 peptides extracted from artificial CSF + 5% rat plasma.
Linearity, Accuracy, and Precision

Figure 4: Representative chromatogram showing basal levels of amyloid β 1-42 extracted from 3 sources of human and 1 source of monkey CSF.N15-labelled internal standards were used for each peptide. Standard curves for all 3 aβ peptides were linear (1/× weighting) from 0.1 to 10 ng/mL in artificial CSF + 5% rat plasma. Basal levels of amyloid β 1-42 extracted from 3 sources of human and 1 source of monkey CSF are shown in Figure 4. Overspike QC samples were prepared in 3 sources of pooled human CSF and 1 source of pooled monkey CSF at 0.2, 0.8, 2, and 6 ng/mL. Accuracy and precision values for all 3 peptides met the regulatory criteria for LC–MS-MS assays. Representative results from QC sample analysis are shown in Table I.
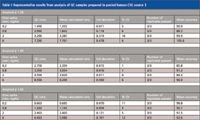
Table I: Representative results from analysis of QC samples prepared in pooled human CSF, source 3
Results and Conclusions
- An SPE-UPLC–MS-MS bioanalytical method was developed and validated for the simultaneous quantification of multiple amyloid β peptides in human and monkey CSF.
- 96 samples can be extracted and ready for injection in < 30 min, providing the sample prep throughput required for pre-clinical and clinical studies.
- The method described herein eliminates time-consuming immunoassays or immunoprecipitation steps for pre-clinical work.
- This approach also allows one assay for the simultaneous measurement of several different amyloid β peptides from a single sample. This single assay provides a high degree of selectivity and specificity in a high-thoughput format while still achieving the high sensitivity required for low level endogeneous amyloid β peptides.
- The use of a single UPLC–MS-MS assay represents a significant advantage over an ELISA assay, which would require multiple assays with multiple antibodies to quantify each of the relevant peptides.
©2010 Waters Corporation. Waters, The Science of What's Possible, ACQUITY UPLC, Oasis, Xevo are trademarks of Waters Corporation.

Waters Corporation
34 Maple Street, Milford, MA 01757
tel. (508) 478-2000; fax (508) 478-1990
Website: www.waters.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















