Extraction of Testosterone and Other Steroid Hormones from Human Plasma Using ISOLUTE® SLE+ 96-Well Plates
A new, simple, and sensitive sample preparation application for the extraction of a range of steroid hormones from human plasma at significantly low levels using ISOLUTE SLE+, an efficient alternative to traditional liquid-liquid extraction (LLE) for bioanalytical sample preparation, providing high analyte recoveries, no emulsion formation, and significantly reduced sample preparation time.
A new, simple, and sensitive sample preparation application for the extraction of a range of steroid hormones from human plasma at significantly low levels using ISOLUTE SLE+, an efficient alternative to traditional liquid-liquid extraction (LLE) for bioanalytical sample preparation, providing high analyte recoveries, no emulsion formation, and significantly reduced sample preparation time.
Steroid hormones are important to help control metabolism, inflammation, immune functions, salt and water balance, and development of sexual characteristics. Steroid hormone testing, especially for testosterone, is becoming more common to screen for a variety of conditions, as well as athlete doping. Sample preparation of plasma samples is always recommended to counteract any matrix effects that may be seen during the analysis of these samples, especially if analysis is being carried out by LC–MS.
Extraction Conditions
This application note outlines the procedure using the 200 µL format (part # 820-0200-P01) but larger formats are available. Method parameters and dilution factors have been optimized to maximize recoveries and minimize ion suppression.
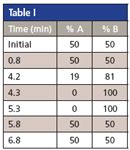
Table I
Sample Pre-treatment: Dilute human plasma (100 µL) 1:1 with HPLC grade water (100 µL).
Sample Load: Load pretreated sample (200 µL) to plate followed by a pulse of vacuum to initiate flow and leave for 5 min to completely absorb.
Analyte Elution: Elute with dichloromethane (1 mL) directly into a deep well collection plate (121-5203). Leave to flow under gravity for 5 min, then apply a short pulse of vacuum to completely remove the aliquot.
Post extraction: Evaporate to dryness at ambient temperature and reconstitute in 50% methanol (aq) (100 µL). Vortex samples to ensure full reconstitution of all analytes.
Additional information: Use of the reconstitution solvent combined with the vortex step ensures there are no issues that may arise from non-specific binding of testosterone and other analytes. All samples were processed using Biotage VacMaster-96 sample processing manifold and evaporated to dryness on a SPE Dry 96.
The extracted samples were run on a Waters Acquity UPLC (Waters Corporation, Milford, Massachusetts) with an Acquity UPLC BEH C18 column (1.7 µm, 100 × 2.1 mm id) (at 40 °C). Gradient elution conditions were optimized using: A) 0.1% (v/v) formic acid (aq) and B) 0.1 % formic acid in methanol at a flow rate of 0.4 mL/min. MS detection was provided by a Premier XE triple quadrupole mass spectrometer (Waters Corporation, Manchester, UK) equipped with an electrospray interface for mass analysis.
Results
Figure 1 demonstrates recoveries >90% with corresponding RSDs below 10% (n = 7) across the range of steroid hormones using this application.
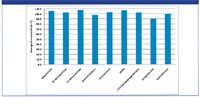
Figure 1: Steroid hormone extraction recoveries from human plasma using ISOLUTE SLE+.
Conclusions
This simplified and efficient extraction method has significant analyte recoveries ranging from 90% to 107%, with LOQs as low as 500 pg/mL. Recovery of lower levels of DHEA (currently LOQ = 100 ng/mL) may be possible using a more specific multiple reaction monitoring (MRM) transition.
References
(1) A. Senior et al., "Extraction of Testosterone and Other Steroid Hormones from Human Plasma Using ISOLUTE® SLE+ 96-Well Plates," Application Note AN 740 (available from www.biotage.com/applications).
Biotage, LLC
10430 Harris Oaks Blvd., Suite C, Charlotte, NC 28269
Email: product_info@biotage.com
Website: www.biotage.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















