Exploring UPLC Selectivity with New High Strength Silica (HSS) Cyano and PFP Stationary Phases
For successful chromatographic method development, it is advantageous to maximize the difference in column selectivity, or "selectivity space" investigated in order to minimize the number of critical analyte co-elutions.
For successful chromatographic method development, it is advantageous to maximize the difference in column selectivity, or "selectivity space" investigated in order to minimize the number of critical analyte co-elutions. This reduces the additional optimization required for that method. Taking full advantage of an expanded selectivity space requires columns with significantly different retention properties. This application note highlights the increase in selectivity options for UPLC (and HPLC), using the new HSS Cyano (CN) and HSS Pentafluorophenyl (PFP) stationary phases. In addition to selectivity differences, columns must be robust and reproducible over time and between particle batches.
Experimental Conditions
All data was collected on an ACQUITY UPLC H-Class System with ACQUITY UPLC PDA Detector. These evaluations utilized ACQUITY HSS and XSelect HSS Columns of various particle sizes and dimensions.
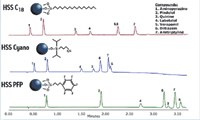
Figure 1: Gradient separations of a basic drug mix on 2.1 à 50 mm ACQUITY columns using a 1.8 μm HSS C18 (top), a 1.8 μm HSS Cyano (middle), and a 1.8 μm HSS PFP (bottom). The mobile phases were 100% methanol and 10 mM ammonium formate in water at pH 3.0. A 3 min gradient from 30% to 85% MeOH, with an additional hold at 85% MeOH was used at a flow rate of 0.4 mL/min. The sample mix was aminopyrazine, pindolol, quinine, labetalol, verapamil, diltiazem, and amitriptyline.
Determining Selectivity
To demonstrate selectivity differences, a gradient separation of a basic drug mix was performed on three different ligand bondings to the high strength silica (HSS) substrate, all under identical conditions (Figure 1). One approach for assessing selectivity differences between columns involves a simple comparison of retention times for a know set of analytes (1). The retention times for each compound of a test mix on one column are used as y-axis data points. The retention times for the test mix on the comparison column are used as the x-axis data points. A linear regression through the x-y data gives the coefficient of determination, R2, which can be used to assess selectivity differences between columns for a given method and set of analytes. Larger deviations from R2= 1 indicate greater differences in selectivity. This is shown for the current data set in Figure 2, revealing R2values of 0.90 and 0.78 for the HSS Cyano and HSS PFP, respectively, for this application. While both columns exhibit different retention behavior relative to the HSS C18, the HSS PFP has the greatest difference in selectivity, with significantly more retention for the basic compounds. This could be particularly useful for samples containing very polar bases.
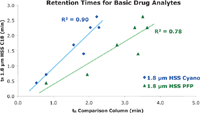
Figure 2: A plot of the analyte retention times for a basic drug mixture on the HSS C18 column (y-axis) vs. the retention times for the same compounds on the HSS Cyano and HSS PFP (x-axis). A linear regression of that data yields coefficients of determination, R2, of 0.90 and 0.78, respectively.
New Selectivity: Reversed-Phase vs. Normal Phase
The chromatographic analysis of steroids is well documented, often utilizing cyano stationary phases (2,3). Because of their moderate polarity, cyano columns have utility for both reversed-phase and normal phase modes of chromatography. This is demonstrated for the HSS Cyano stationary phase with the separation of a mixture of structurally close steroids under both reversed-phase and normal phase conditions, with significantly different selectivities observed between the methods (Figure 3).
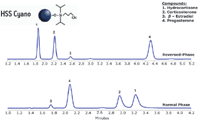
Figure 3: Isocratic separations of a steroid mix on a 3.5 μm XSelect HSS Cyano, 2.1 à 100 mm column under reversed-phase (top) and normal phase (bottom) conditions. The flow rate was 0.19 mL/min with and isocratic mix of 50:50 acetonitrile/water (for reversed-phase) and 82:12.8:5.2 hexane/IPA/dichloromethane (for normal phase). The sample mix was hydrocortisone, corticosterone, β-estradiol, and progesterone. Sample diluents were a 50:50 mixture of acetonitrile/water (for reversed-phase) or hexane/IPA (for normal phase).
Scalability and Method Transfer
The ability to scale between UPLC and HPLC methods requires batch-to-batch reproducibility for all particle sizes. Historically, chromatography using PFP columns has suffered from the challenges of producing reproducible PFP stationary phases. Figure 4 demonstrates scaling between different particle sizes and column dimensions, using a HILIC gradient separation of basic analytes. Column configurations were selected to maintain approximately the same column length to particle size ratio, (L/dp).
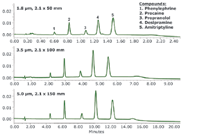
Figure 4: Demonstration of scaling between HSS PFP columns of different dimensions and particle sizes (1.8 μm, 2.1 μ50 mm [top], 3.5 μm, 2.1 μ100 mm [middle], and 5.0 μm, 2.1 μ150 mm [bottom]) using a gradient separation of basic drugs under HILIC conditions. The mobile phases were 100% acetonitrile and 10 mM ammonium formate in water at pH 3.0. A gradient of 75% to 55% acetonitrile was used at 0.8 mL/min for the 1.8 μm particle size configuration. All additional flow rates and gradient times were scaled appropriately to account for the differences in particle size and column dimension. The sample mix was phenylephrine, procaine, propranolol, desipramine, and amitriptyline.
Conclusions
The two new additions to the ACQUITY HSS and XSelect families of columns offer new choices for chromatographic selectivity. The available 1.8 μm, 2.5 μm, 3.5 μm, and 5.0 μm particle sizes, in combination with excellent batch-to-batch reproducibility, enables scaling between UPLC and HPLC applications. The versatility of these stationary phases enables modes of chromatography beyond reversed-phase, including normal phase and HILIC. The HSS PFP exhibits increased retention behavior for very polar basic compounds making it particularly useful for pharmaceutical compounds.
References
(1) Uwe Neue, John E. O'Gara, and Alberto Méndez, J. Chromatogr A 1127, 161–174 (2006).
(2) Pirkko Volin, J. Chromatogr. B 671, 319–340 (1995).
(3) H.L.J. Makin and D.B. Gower (Eds.), Steroid Analysis – 2nd Ed. (Springer, 2009, ISBN: 978-1-4020-9774-4).
©2011 Waters. ACQUITY UPLC, UPLC, XSelect are copyright of Waters Corporation.
Waters Corporation
34 Maple Street, Milford, MA 01757
tel. (508) 478-2000, fax (508) 478-1990
Website: www.waters.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















