Evaluation of QuECheRS, Cartridge SPE Cleanup, and Gas Chromatography Time-of-Flight Mass Spectrometry for the Analysis of Pesticides in Dietary Supplements
LCGC North America
The authors evaluate a method for detecting pesticide residues in dandelion root powder.
The FDA is responsible for regulation of dietary supplements as foodstuffs through the 1994 Dietary Supplement Health and Education Act (DSHEA) amendment to the Food Drug and Cosmetic Act (FDCA). Product standardized practices were set, but compliance was voluntary and no legal requirement existed for manufacturers. Due to public and industry concern, in 2003, the FDA proposed requiring dietary supplement manufacturing to adhere to current good manufacturing practices (cGMPs). The final rule was issued in June 2007 and is in full effect June 2010 (1). Basic GMPs require implementing comprehensive procedures to ensure product quality and safety. Because many dietary supplements are largely derived from botanical sources, they must be tested for pesticide contaminants in order to meet cGMP regulations. As a result of this requirement, labs are working to develop and validate methods, an endeavor that is complicated by the wide range of pesticides and matrices to be tested.
Although other researchers have used established methods like those found in the FDA Pesticide Analytical Manual (PAM) (2) as an alternative, we developed a QuEChERS (quick, easy, cheap, effective, rugged, and safe)-based method for analyzing pesticides in dietary supplements. QuEChERS is an approach developed by Anastassiades and colleagues (3) as a simple, rapid, effective, yet inexpensive way to extract pesticide residues from fruits and vegetables, followed by a novel dispersive solid-phase extraction (dSPE) cleanup of the extract. Because of these benefits, the approach has become popular and has been expanded to include numerous other matrices. We chose QuEChERS as an alternative to PAM-based methods because of its speed, simplicity, and low solvent use, as well as its ability to produce good extraction efficiencies for relatively polar pesticides (3).

Experimental
Chemicals and materials: QuEChERS extraction and dSPE tubes, QuEChERS internal and quality control standards, and cartridge SPE tubes (cSPE) were from Restek Corporation (Bellefonte, Pennsylvania). The 46-pesticide mix, representing different chemical classes that have been reported previously in dietary supplements (4–6), was a custom standard produced by Restek Analytical Reference Materials group (Bellefonte, Pennsylvania).
Sample preparation:
Sample wetting: Typically, QuEChERS methods use 10–15 g of material and are ideal for commodities with high water content (>80%). Therefore, to prepare for a QuEChERS extraction with a dry commodity, it is critical to use a reduced amount of material and wet it with water prior to extraction. In this work, 1 g of dietary supplement powder was combined with 9 mL of water. After shaking to mix well, the wetted supplement was fortified with 200 μL of a 2 ng/μL pesticides spiking solution resulting in a 400-ng/g spike level, relative to the original commodity. Also, 100 μL of QuEChERS Internal Standard Mix for GC–MS analysis was added. The sample was allowed to soak for 2 h.
QuEChERS extraction: The EN 15662 QuEChERS method was used for sample extraction (7). A 10-mL volume of acetonitrile was added to the wetted sample. After a 1-min shake, buffering extraction salts (Q-sep Q110, Restek) containing 4 g magnesium sulfate, 1 g sodium chloride, 1 g trisodium citrate dihydrate, and 0.5 g disodium hydrogen citrate sesquihydrate were added. Following another 1-min shake, the sample was centrifuged for 5 min at 3000 U/min with a centrifuge (Q-sep 3000, Restek). Lastly, 5 μL of a quality control standard containing anthracene was added to a 1-mL aliquot of extract to monitor for potential losses of planar compounds to a graphitized carbon black during cleanup.
Extract cleanup: Based upon preliminary studies, we knew that while the extraction part of QuEChERS would be successful, the dSPE cleanup step probably did not have the capacity to handle the matrix complexity and amount of most dietary supplements. Thus, we compared dSPE to a cSPE cleanup and established a procedure that uses a QuEChERS extraction, cSPE cleanup, and GC–TOF-MS for determinations of 46 pesticides in dandelion root powder.
Two approaches were explored for extract cleanup: dSPE and cSPE. For dSPE, 1 mL of extract was added to a Q210 dSPE tube containing 150 mg of magnesium sulfate and 25 mg primary secondary amine (PSA), shaken for 2 min, and then centrifuged for 5 min. The resulting final extract was then analyzed by GC–TOF-MS.
For cSPE cleanup (8), 1 mL of extract was processed with a 6-mL Combo SPE cartridge (Resprep, Restek), which is designed for pesticide residue cleanup and contains 500 mg of a form of graphitized carbon black and 500 mg PSA. To prepare the SPE cartridge, approximately 300 mg of magnesium sulfate was first added; then the cartridge was rinsed with 20 mL of 3:1 acetonitrile–toluene, which was discarded. For cleanup, 1 mL of extract was loaded onto the prepared cartridge and then eluted with 50 mL 3:1 acetonitrile–toluene. The eluent was then evaporated and solvent exchanged using dry nitrogen gas and a 35–40 °C water bath. Evaporation was allowed to proceed until approximately 0.5–1 mL eluent was left, at which point about 3 mL of toluene was added. The mixture was evaporated to just under 0.5 mL, and then the evaporation vessel was rinsed with toluene to bring the sample to a final volume of 0.5 mL. The resulting final extract was then analyzed by GC–TOF-MS.
Matrix-matched standards: Matrix-matched standards were prepared at 80 pg/μL, as 80 pg/μL is the expected final concentration in extract of the 400-ng/g matrix spikes (assuming 100% recoveries). Matrix-matched standards were prepared by adding standard solution to the final extract (postcleanup) from a control sample. Actual recoveries were calculated by comparing peak areas for fortified samples that were extracted and cleaned up, to areas of a matrix-matched standard, using the internal standard quantification method with PCB 52 from the internal standard mix added before extraction.
GC-TOF-MS analysis: A LECO Pegasus III GC–TOFMS system was used and all data were processed with software from LECO (ChromaTOF, LECO Corporation, Saint Joseph, Michigan). GC was performed using a 30 m × 0.25 mm, 0.25-μm df column (Rxi-5Sil MS, Restek) with a constant flow of helium at 1.5 mL/min and a fast autosampler splitless injection of 1 μL, purge valve time of 1.5 min, into a 5-mm single gooseneck liner with wool (Restek). The inlet temperature was 250 °C and the GC oven program began with a temperature of 90 °C (1.5 min) followed by an increase at 8 °C/min to 340 °C. Total run time was 32.75 min. Electron ionization at 70 eV was used with a source temperature of 225 °C. Data acquisition was from 45 to 550 u at an acquisition rate of 5 spectra/s.
Results and Discussion:
One aspect of this investigation was to compare the applicability of two sample cleanup methods, dSPE and cSPE, for QuEChERS extracts of pesticides in dietary supplements. Several formulations of dSPE were tested having different combinations and varying amounts of PSA, C18 and GCB sorbents. While dSPE has the advantage of improved speed and less solvent usage, it does not have the sorbent capacity to adequately clean up these samples (Figure 1). Figure 1 shows a comparison of the effectiveness of dSPE and cSPE to remove common matrix compounds like fatty acids, sugars and other co-extractives. The blue plot on the left side shows the fatty acids of dandelion root processed with dSPE, while the green plot is that of dandelion root processed by cSPE. Because cSPE uses more sorbent, it is a better choice for dietary supplements (and other complex samples, for example, spices, essential oils) as it can remove a higher amount of matrix components, such as fatty acids, sugars, and pigments. QuEChERS methods developed for dietary supplements of botanical origin can benefit from the extra sorbent capacity of cSPE, which reduces GC inlet–column contamination and chromatographic interference from complex botanical matrices.

Figure 1: Comparison of sugars and fatty acids removal by extracted ion chromatograms of m/z 60. Left: dandelion root processed with dSPE. Right: dandelion root processed with the cSPE method described in the Experimental section.
Even with effective extraction and cleanup techniques, dietary supplements can be challenging to analyze due to their complexity. Coelutions are common and pesticide residues can be overwhelmed by abundant matrix compounds not only qualitatively, but also by interfering with quantitation masses. Figure 2 plots the total ion chromatogram (TIC) and extracted ion m/z 312 corresponding to the quantitation mass for carfentrazone ethyl. It is clear that target pesticide signals can be obscured in the TIC. The software mentioned earlier was used to identify target pesticides by comparison with empirical reference spectra, after automatic peak find and spectral deconvolution algorithms, as well as performing calibration and quantification. TOF-MS makes this powerful data processing possible by providing fast acquisition rates and unbiased mass spectra, and by having picogram-level sensitivity in full mass range mode, which also allows the potential for finding nontarget pesticides. An alternate GC–MS approach for targeted pesticides in dietary supplements would be to use selected ion monitoring (SIM) with a typical quadrupole mass spectrometer.

Figure 2: Total ion chromatogram (green) and extracted ion chromatogram of m/z 312 corresponding to the quantitation ion for carfentrazone ethyl (blue). The inset shows the enlarged carfentrazone ethyl peak with a quantitation signal-to-noise value of 105.
Recoveries: Overall, the combination of QuEChERS extraction, cSPE cleanup, and GC–TOF-MS used in this method produced good recoveries for most compounds tested (Table I). Although early eluted compounds trended toward slightly lower recoveries, most analytes, including more polar compounds, showed excellent recoveries. The potential for good recoveries of polar pesticides is a major advantage of QuEChERS methods. This difference is due to the use of a polar extraction solvent, acetonitrile rather than petroleum ether (hexanes), which is used in PAM 303, for example. The lower recoveries of early eluted compounds can be due to evaporative loss during concentration steps, due to their higher volatility. Additionally, in the case of planar compounds (for example, the chlorinated aromatics), reduced recoveries can be due to interaction with the graphitized carbon black sorbent used to remove pigments and other matrix compounds, although the planar quality control standard, anthracene, did not show drastic losses during cSPE. Overall, the chromatography and recovery results seen for a broad range of pesticides in dandelion root demonstrate the utility of the QuEChERS approach for dietary supplement testing.
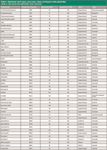
Table I: Retention time data, recoveries, class, and type of the pesticides used in this work for dandelion root extracts.
Conclusion
Demonstrated here is a QuEChERS extraction, cartridge SPE cleanup, and GC–TOF-MS approach that helps accomplish the pesticide testing now required for dietary supplements. The basic methodology presented here for dandelion root can be modified for other analytes and matrices and illustrates the advantages of the QuEChERS approach for labs developing cGMP methods. Analytical benefits include reduced interferences and good recoveries, even of polar compounds. Other benefits include an overall savings of both materials and sample preparation time compared to the PAM-based methods, and better expected reproducibility due to the straightforward procedure with fewer manual preparations.
Acknowledgment
The authors would like to acknowledge LECO Corporation for the generous use of their instrument.
Julie Kowalski, Michelle Misselwitz, Jason Thomas, and Jack Cochran are with Restek Corporation, Bellefonte, Pennsylvania.
References
(1) US Food and Drug Administration, Current Good Manufacturing Practice in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements, Docket No. 1996N-0417 (formerly No. 96N-0417), CFSAN 200441 (2007) 34752.
(2) US Food and Drug Administration, Pesticide Analytical Manual, Volume I: Multiresidue Methods, 3rd ed. 1994.
(3) M. Anastassiades, S.J. Lehotay, D. Stajnbaher, and F.J. Schenck, J. AOAC Int. 86, 412 (2003).
(4) J.W. Wong, M.S. Wirtz, M.K. Hennessy, F.J. Schenck, A.J. Krynitsky, and S.G. Capar, Acta Hort. (ISHS) 720, 113–128 (2006).
(5) J.W. Wong, M.K. Hennessy, D. G. Hayward, A. J. Krynitsky, I. Cassias, and F.J. Schenck, J. Agric. Food Chem. 55, 1117–1128 (2007).
(6) D. G. Hayward and J.W. Wong Anal. Chem. 81, 5716–5723 (2009).
(7) Foods of Plant Origin — Determination of Pesticide Residues Using GC–MS and/or LC–MS/MS Following Acetonitrile Extraction/Partitioning and Clean-up by Dispersive SPE (QuEChERS-method). (EN 15662 Version 2008.)
(8) M. Okihashi, Y. Kitagawa, K. Akutsu, H. Obana, and Y. Tanaka, J. Pestic. Sci. 30, 368–377 (2005).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











