An Empirical Evaluation of HILIC and Monolithic Columns for SFC Applications
Separation of polar compounds by conventional reverse phase chromatography can be challenging due to their poor retention. Other HPLC approaches include normal phase chromatography (NPC) and its variation, hydrophilic interaction chromatography (HILIC).
Lakshmi Subbarao, Jacquelyn Cole, and Rui Chen, TharSFC, a Waters Company
Separation of polar compounds by conventional reverse phase chromatography can be challenging due to their poor retention. Other HPLC approaches include normal phase chromatography (NPC) and its variation, hydrophilic interaction chromatography (HILIC). However, NPC typically suffers from solubility issues, poor reproducibility, and low MS detection sensitivity. As for HILIC, reasonable retention can only be realized when a high percentage of organic solvent (>70%), typically acetonitrile, is used.
More recently, supercritical fluid chromatography (SFC) has become an attractive alternative for the separation of polar compounds (1). Similar to NPC and HILIC, SFC generally employs polar stationary phases and a less polar mobile phase, supercritical CO2 in combination with methanol. Owing to the speed advantage as a result of the inherent low viscosity of supercritical CO2, SFC has been used in high throughput analysis of drug-like compounds and bio-analysis (2–3). To this end, monolithic columns are of interest in SFC applications for their potential to further improve throughput.
In this application note, we present our empirical evaluation on three different forms of silica columns for SFC applications: a particle packed silica column, an ethylene-bridged hybrid (BEH) silica column marketed as a HILIC column, and a monolithic silica column.
Experimental
All chemicals were obtained from Sigma Aldrich (St. Louis, MO) and used as received. Stock solutions of 1 mg/mL were prepared for each compound as well as for the mixtures in 70:30 (v/v) methanol/water.
All experiments were conducted using a Waters TharSFC Method Station II controlled by Empower® software. A Spherisorb® particle packed silica column and an XBridge™ HILIC column (both 4.6 X 100 mm, 5 µm) were purchased from Waters (Milford, MA). An Onyx™ monolithic silica column (4.6 X 100 mm) was purchased from Phenomenex (Torrance, CA).
All experiments were run under the following conditions unless otherwise specified: flow rate: 4 mL/min; back pressure: 150 bar; temperature: 40 °C; injection volume: 5 µL (full loop); wavelength scan range: 220 to 300 nm; modifier: methanol; gradient: 5% to 40% in 5 min, 40% for 1 min, 40% to 5% in 2 min, and held at 5% for 2 min.
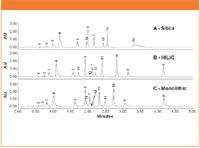
Figure 1: SFC chromatograms at 254 nm obtained under gradient conditions. Test compounds are listed below. Acids: 1. Ibuprofen; 2. Fenoprofen; 3. Naproxen; 4. Ketoprofen. Bases: 5. Theophylline; 6. Thymine; 7. Uracil; 8. Adenine; 9. Cytosine; 10. 6-amino-1,3-dimethyluracil. Neutral polar compound: 11. Sulfamethoxazole. Neutral non-polar compounds: 12. Estradiol; 13. Cortisone; 14. Amcinonide.
Results and Discussion
Figure 1 shows the SFC chromatograms obtained under the described gradient condition for each column. In summary, 12 of the 14 compounds eluted off the particle packed silica column and all 14 compounds eluted off the HILIC and monolithic columns. There appears to be more retention on the particle packed silica column as compared to the HILIC and monolithic columns. Two basic compounds, adenine and cytosine (peaks 8 and 9, respectively) did not elute off the particle packed silica column. In addition, compound 10, 6-amino-1,3-dimethyl-uracil, displayed significant tailing on the particle packed silica column, compared to the other two columns. This is possibly due to the presence of excessive acidic silanol groups on the surface of the particle packed silica column, and consequently, excessive retention for basic compounds. In SFC, it is a common practice to add small tertiary amines, such as dimethylethylamine and isopropyl amine, to shorten the retention time and improve the peak shape by blocking the active silanol sites; and a thorough column flushing afterwards is highly recommended. Both the HILIC and monolithic columns, on the other hand, demonstrated reasonable retention and more symmetrical peak shape, especially for the BEH HILIC column, owing to the more controlled surface chemistry. It is speculated that the ethylene bridges within the silica matrix not only provide improved chemical and mechanical stability, but also effectively reduce the number of active silanol sites.
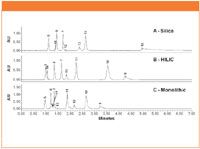
Figure 2: SFC chromatograms at 254 nm obtained under isocratic conditions (10% methanol) for 9 selected compounds. Compounds are listed here. 5. Theophylline; 6. Thymine; 7. Uracil; 8. Adenine; 10. 6-amino-1,3-dimethyluracil; 11. Sulfamethoxazole; 12. Estradiol; 13. Cortisone; and 14. Amcinonide.
A mixture consisting of compounds 5 through 8 and 10 through 14 was then injected under isocratic conditions (10% methanol) on all three columns. The resulting chromatograms are shown in Figure 2. Similar to the gradient experiments, 8 of the 9 compounds eluted off the particle packed silica column, whereas all 9 compounds eluted off the HILIC and monolithic columns. Compared to the particle packed silica and HILIC columns, the monolithic column displayed a somewhat different selectivity, especially for relatively bulky compounds, such as cortisone (compound 13) and amcinonide (compound 14). The increased retention is likely due to steric hindrance that impedes their passage through the deep mesopores of the monolithic column. Capacity factor (k'), plate number (at w½), and USP tailing factor for baseline resolved peaks were calculated and are listed in Table I. In summary, under SFC conditions, both HILIC and monolithic columns offered sufficient retention for all tested compounds, including polar basic compounds. The HILIC column offered comparable plate numbers with the monolithic column and more symmetrical peak shapes.

Table I: Selected system suitability parameters. S: Spherisorb® silica; H: XBridge⢠HILIC; and M: Onyx⢠monolithic.
Conclusions
A mixture of 14 compounds, including 6 polar basic compounds, were baseline resolved on the HILIC column and partially resolved on the monolithic column, under generic SFC gradient conditions without the addition of additives. The monolithic column displayed a somewhat different selectivity compared to the particle packed and HILIC columns, which can be ascribed to steric hindrance from the analytes. Both XBridge™ HILIC and Onyx™ monolithic silica based columns have great potential for routine use in SFC applications.
References
(1) Y. Zhao, P. Sandra, G. Woo, S. Thomas, K. Gahm, D. Semin, LCGC Europe, 17 (2004) 224–238.
(2) B.J. Bolanos, M. Ventura, M. Greig, J. Comb. Chem. 5 (2003) 451–455.
(3) S.H. Hoke, II, J.A. Tomlinson, II, R.D. Bolden, K.L. Morand, J.D. Pinkston, K.R. Wehmeyer, Anal. Chem. 73 (2001) 3083–3088.

TharSFC, a Waters Company
575 Epsilon Drive, Suite 100, Pittsburgh, PA 15238
tel. (412)967-5665; fax (412)967-9446
Email: info@tharsfc.com Website: www.waters.com/sfc

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)















