Efficient Sample Preparation and High-Capacity Columns for Successful Food and Beverage Analysis
When it comes to the topic of foods and beverages, consumers want to know the details of the ingredients in the products that they purchase. Whether due to dietary restrictions or health concerns, this information can be critical and must consequently be reliable. It is therefore not surprising that the regulations in this field have become ever stricter to ensure high standards of product quality. Ion chromatography (IC) is a versatile tool that can analyze multiple regulated components in the same run. Two of the most essential factors for successful IC analysis of food and beverage samples are discussed in this article.
Food quality and labelling must comply with consumer needs as well as increasingly stricter regulations (for example, EU regulation 1169/2011, US 21 CFR Part 101) (1,2). Robust, reliable, and fast determination of food quality parameters is therefore indispensable.
Nutritionist with fresh products on light background and images of different vegetables and fruits, closeup. Healthy eating | Image Credit: © New Africa - stock.adobe.com

Food and beverage analysis is challenging—not only due to the vast range of analytes but also because of the sample matrix. These samples are complex and heterogeneous, with different physical properties and a wide variety of components that can interfere with the analytical procedure (3).
Overcoming the challenges of food and beverage analysis is possible with ion chromatography (IC). IC is a robust and straightforward analytical technique that can analyze multiple components within a single run. It has many benefits for food and beverage analysis, such as:
- It is straightforward and easy to use, with low instrument and operating costs.
- It is environmentally friendly due to low chemical usage.
- It is flexible—several detectors can be combined for comprehensive analysis of anions, cations, carbohydrates, and more.
- IC with amperometric detection is selective, sensitive, and quick, with very low detection limits.
Nevertheless, two critical points must be considered to guarantee accurate analysis of food samples:
- An appropriate sample preparation prior to the analysis;
- A suitable column, particularly regarding properties such as column capacity and selectivity.
Sample preparation prolongs instrumental and column lifetime and reduces interfering components. The column capacity is essential for the separation efficiency of the remaining sample matrix from the analytes of interest, and thus the final detection capabilities.
The Role of Sample Preparation
Instrumental analysis of foods requires sample preparation to guarantee a long system lifetime along with reliability, reproducibility, and accuracy of the results (4–7). Foods and beverages are complex heterogenous mixtures (3). Such matrices can be aggressive and degrade the analytical system, leading to precipitation issues or clogging; they may even interfere with the analytes of interest during analysis.
Multiple procedures exist to prepare samples for analysis and/or improve the accuracy of the results. These include routine steps such as weighing, grinding, homogenization, mixing, dilution, heating, or cooling, procedures for preservation, as well as clean-up techniques such as filtration, centrifugation, digestion, extraction, or precipitation (7).
Most sample preparation practices are performed manually and offline, accounting for 60–80% of the laboratory effort and operating costs (3). This practice can be prone to human error depending on the complexity of the sample treatment, and results may vary between operators (3).
While particles are easily removed by filtration or centrifugation (5), fats and proteins require more specific treatments. Traditionally, Carrez clarification is used to remove proteins and fats from food samples via precipitation (8). However, this manual procedure requires a significant amount of work and specialized chemicals.
Automated alternatives are faster, more cost‑effective, and greener (3). These approaches have been widely adopted by the scientific community and are used in routine analysis for multiple applications (9–12).
Influence of the Column Capacity
The separation column plays a crucial role in the quality of each analysis (13). For the determination of anions, cations, and carbohydrates, the respective analytical columns are typically made from densely packed spherical, polymeric particles.
The base materials are frequently made from polystyrene-divinylbenzene copolymer (PSDVB) or polyvinyl alcohol (PVA) (14,15). This material impacts the compatibility of the column with the sample matrix, as it may exhibit secondary, undesirable interactions with matrix constituents (16).
Column fouling is a risk when analyzing foods and beverages. If hydrophobic constituents from the sample stick to the column material, this can result in a pressure increase and column blockage. PVA materials are less prone to this because they do not allow for many additional interactions, while PSDVB particles contain many conjugated electrons that promote these interactions. Recent advances in PSDVB column materials show that such interactions can be minimized by properly coating the PSDVB base material (17).
The base material not only affects these secondary interactions—it also impacts the column stability against extreme pH or temperature changes. With regard to stability, PSDVB materials are considered superior to PVA materials because they can withstand the full pH range (0–14).
Surface modification of the base material is responsible for the ion-exchange mechanism and the retention of ions on the column. The type of functional group linked to the particle surface controls the selectivity of the stationary phase and is responsible for the elution order of the analytes in the chromatogram (18). In food samples, many low-molecular-weight organic acids must be adequately separated from the inorganic anions, though not all stationary phases provide the required selectivity to achieve this (19).
The number and density of ion-exchange groups is reflected by the capacity of the column. High-capacity columns are recommended when analyzing complex matrices. The benefits of using a high‑capacity column are twofold:
- Ions of interest are strongly retained while non-binding constituents can be flushed out. This lowers the risk of interferences.
- A high-capacity column with many ion-exchange groups ensures that the separation column will not overload, even when the sample contains a large concentration of multiple ions.
The following examples show the benefits of sample preparation paired with a suitable high‑capacity column for IC analysis of foodstuffs.
Drinking Water Analysis: Not Always Easy
Drinking water is essential for many vital functions (20). It contains nutrients, acts as a solvent, ensures molecular transport, fulfils structural tasks, and is involved in many life‑supporting reactions (20). Drinking water must be clean, and safe drinking water is considered a human right (21). However, it is estimated that up to 13% of the global population does not have access to safe drinking water (22,23).
Microbial contamination has a large impact on drinking water quality, but the presence of chemicals can also pose serious health risks (24). To serve public health, comprehensive and regular monitoring of drinking water quality is required in many countries following regulations from the WHO (21), EPA (25), European Union (22), or local regulations (as described in a WHO report [22]). Appropriate and reliable analytical techniques are essential to fulfil these requirements. Ion chromatography is a suitable technique, as it can measure important quality parameters beside toxic disinfection by-products, as described in EPA 300.1 A and B (26).
Measuring inorganic anions in drinking water may seem simple. Sample anions are separated with an anion-exchange column before passing through the detector. However, depending on the water source, large amounts of carbonate may be present and are also retained. If the carbonate peak elutes close to another anion, it may cause a disturbance. Peak shape will be negatively impacted, and the peak can even split. Figure 1(a) shows this undesirable behaviour: With increasing concentrations of bicarbonate, the nearby chloride and nitrite peaks become broader. The consequences are worse for nitrite; at low bicarbonate concentrations, a shoulder appears, and at high bicarbonate levels the nitrite peak splits into two due to local column overloading. This leads to erroneous results.
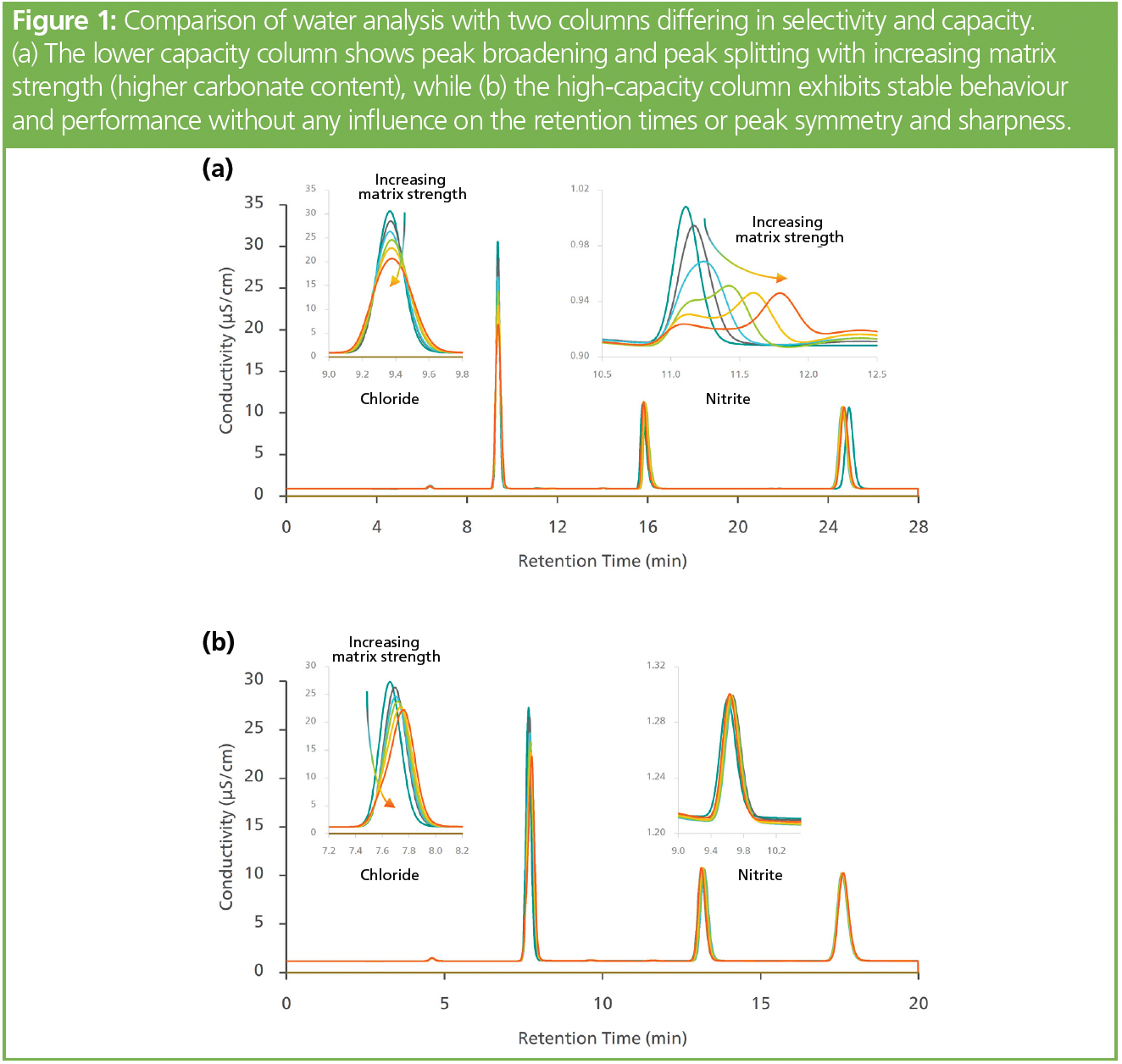
Column selectivity and capacity are important factors to ensure reliable results. The appropriate selectivity can shift the elution of the carbonate peak away from the other anions. A high ion‑exchange capacity guarantees that the column is not overloaded, even when injecting highly loaded samples. Figure 1(b) shows an example using a high‑capacity separation column for such situations (17,27). Independent of the bicarbonate in the sample, the peaks remained sharp and symmetric, with no signs of column overloading.
Experimental:
Analysis Parameters for IC Measurement of Water with High Carbonate Content: Column: Metrosep A Supp 19 - 150/4.0, Metrosep A Supp 19 Guard/4.0; eluent: 8.0 mmol/L Na2CO3, 0.25 mmol/L NaHCO3; eluent flow rate: 0.70 mL/min; column temperature: 30 °C; injection volume: 40 μL; IC: 930 Compact IC Flex Oven/Ses/PP/Deg, 858 IC Sample Processor; detector: IC Conductivity Detector; software: MagIC Net (all instruments are from Metrohm).
Sulphite Determination via Two Methods
Food additives include preservatives, flavouring and colouring agents, and emulsifiers (28,29). However, some are considered harmful (29–32). Food additives are generally regulated, for example, by CODEX alimentarius or by the European Commission in EU Regulation (EC) No 1333/2008 (33,34).
Sulphite is primarily used in food to stabilize colour or flavour (35–37). It has been known to induce asthmatic or anaphylactic reactions, dermatitis, abdominal pain, and diarrhoea (35). Acceptable daily intake (ADI) recommendations for sulphite have been set by the European Food and Safety Authority Panel at 0.7 mg/kg body weight (38).
IC has been established as a routine method for sulphite determination and a proven alternative to high performance liquid chromatography (HPLC) or the traditional Monier‑Williams approach (AOAC 990.28) (28,29,35).
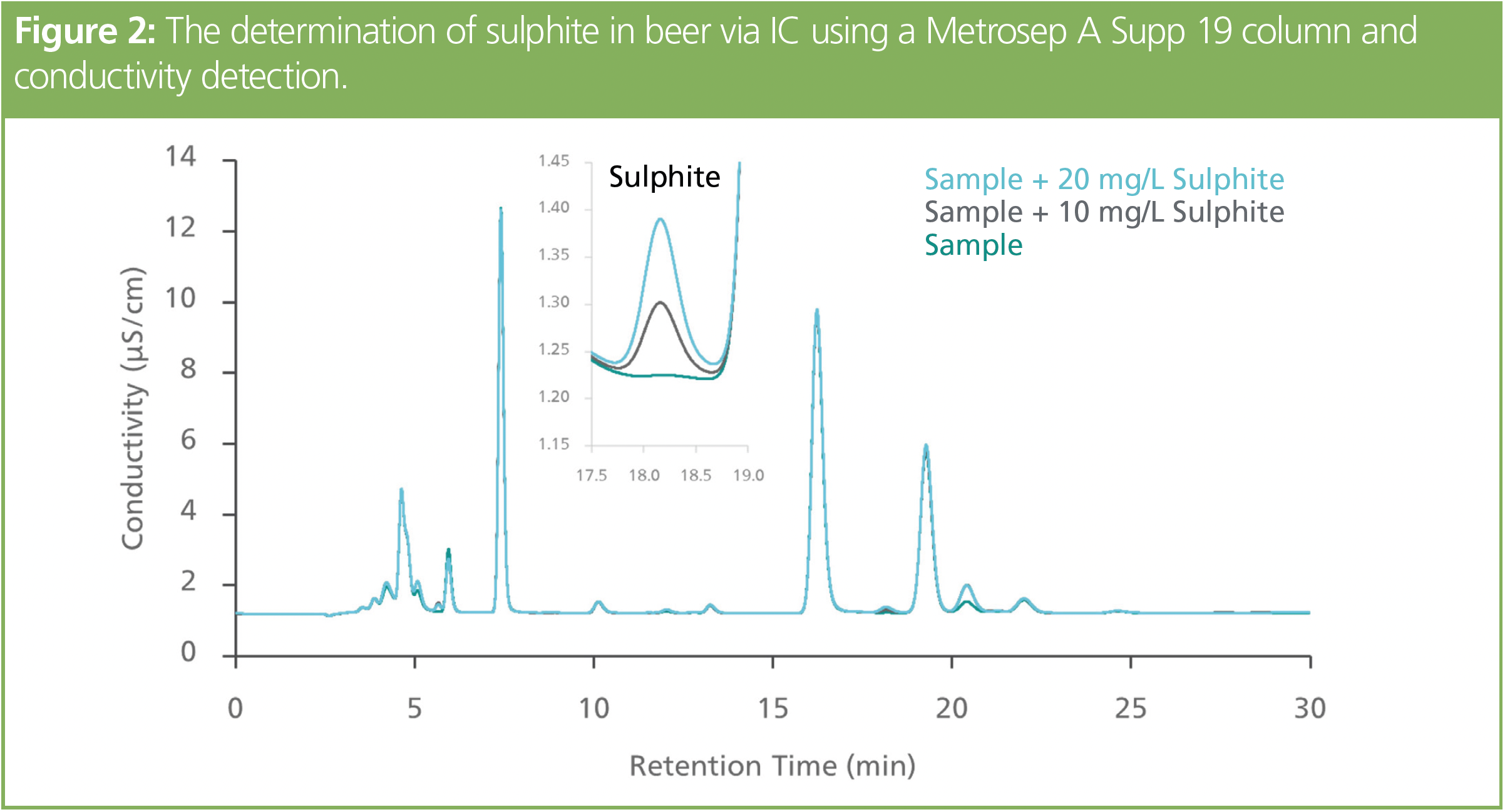
Sulphite measurement in food is simple using IC with conductivity detection. Figure 2 shows an example of sulphite measured in a beer sample using a high-capacity anion-exchange column for the reasons explained in the previous example. The sulphite peak was well separated from all other components and was accurately determined down to 10 mg/L.
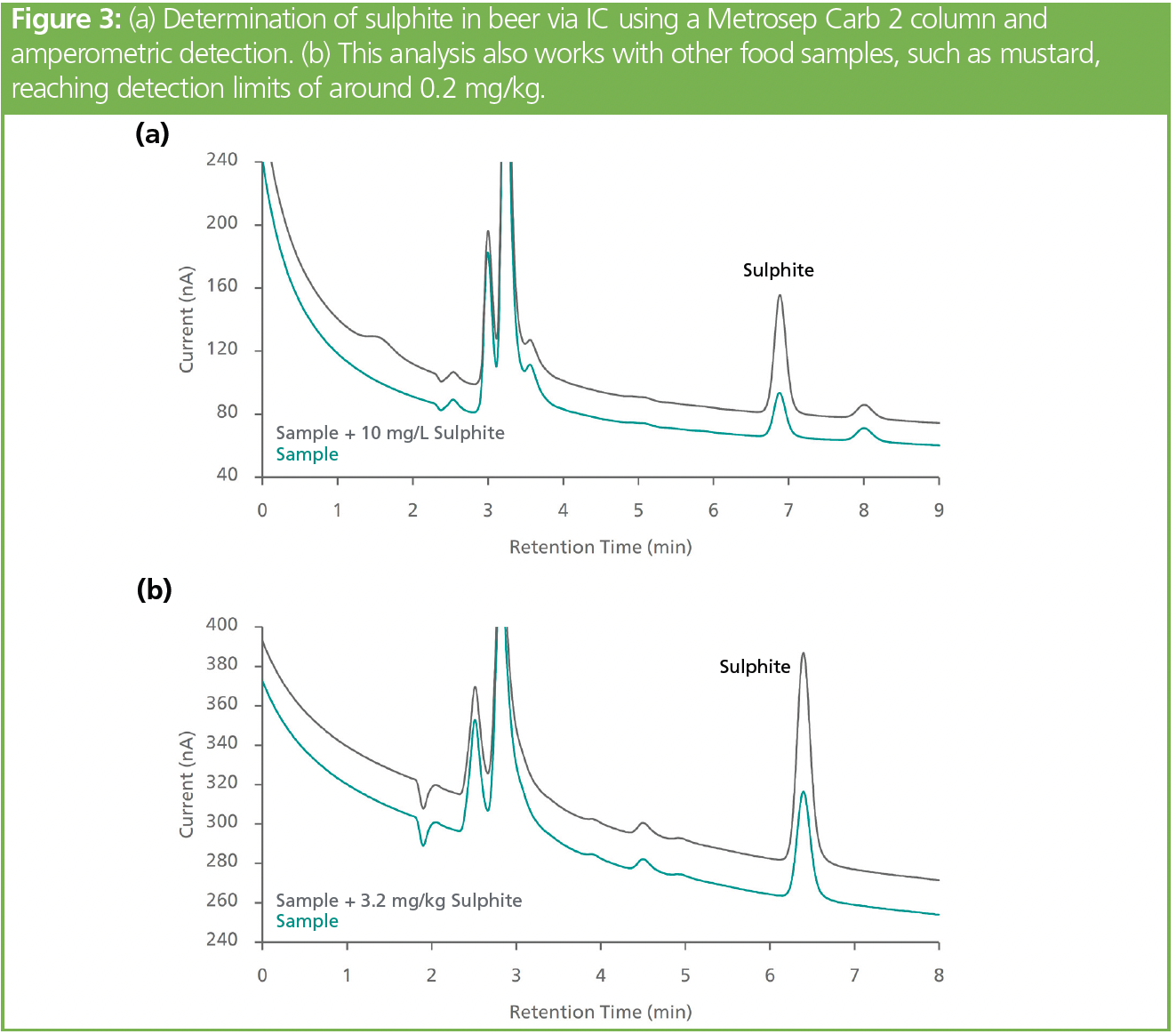
Amperometric detection can reach far lower detection limits (0.2 mg/kg sulphite). Sulphite determination in alkaline food extracts (AOAC 990.31) has limitations for dark-coloured foods (electrode fouling, manual polishing needed). An innovative approach using DC mode and a potential sweep (patent filed, EP3786628A1) after every analysis can minimize manual cleaning and increase analytical stability (39,40).
The alkaline eluents required for this method limit the amount of eligible separation columns. A high-capacity column that can withstand pH values from 0 to 14 is well suited for this application (Figure 3).
Experimental:
Analysis Parameters for Sulphite Determination Using Conductivity Detection: Column: Metrosep A Supp 19 - 150/4.0, Metrosep A Supp 10 Guard HC/4.0; eluent: 8.0 mmol/L Na2CO3, 0.25 mmol/L NaHCO3; eluent flow rate: 0.75 mL/min; column temperature: 45 °C; injection volume: 100 μL; dilution: 1:20; IC: 930 Compact IC Flex Oven/ Ses/PP/Deg, 858 IC Sample Processor; detector: IC conductivity detector; sample stabilization: 2% isopropanol; software: MagIC Net (all instruments are from Metrohm).
Analysis Parameters for Sulphite Determination Using Amperometric Detection: Column: Metrosep Carb 2 - 150/4.0, Metrosep Carb 2 Guard/4.0; eluent: 300 mmol/L NaOH, 300 mmol/L CH3COONa, CO2-free ultrapure water; eluent flow rate: 0.5 mL/min; temperature: 35 °C; injection volume: 3 μL; dilution: 1:10; IC: 930 Compact IC Flex Oven/Deg, 889 IC Sample Center – Cool; detector: IC Amperometric Detector; Wall-Jet Cell; working electrode: Au working electrode, 3 mm; reference electrode: Ag/AgCl; potential: 300 mV (DC mode), potential sweep after analysis (39,40); sample stabilization: 1.00 mmol/L CH2O, 0.20 mmol/L NaOH; software: MagIC Net (all instruments are from Metrohm).
Conclusion
High-capacity separation columns with the appropriate selectivity are critical for successful IC analysis of food and beverage samples. The large number of ion-exchange groups guarantees that the column is not overloaded and that accurate results can be obtained. Sample matrix effects are significantly reduced, resulting in excellent repeatability of analyte retention times.
Sample preparation, a necessity for food and beverage samples, can be extremely tedious and error-prone when performed manually. Combining a high-capacity ion-exchange column with dedicated and automated in-line sample preparation tools (dialysis, filtration, or dilution) establishes optimal analysis conditions, producing more reliable results.
References
(1) The European Parliament and the Council of the European Union (2011), Regulation (EU) No 1169/2011 of the European Parliament and the Council of 25 October 2011 on the Provision of Food Information to Consumers, Official Journal of the European Union, 22.11.2011, L304/18-63, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R1169 (accessed 2023-05-24).
(2) FDA, CFR (Code of Federal Regulations) Title 21, Food and Drugs Chapter I - Food Administration Department of Health and Human Services, Subchapter B - Food for Human Consumption, Part 101 Food Labeling, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=101 (accessed 2023-05-24).
(3) Ramos, L. Basics and Advances in Sampling and Sample Preparation. In Chemical Analysis of Food: Techniques and Applications; Elsevier, 2012; pp 3–24.
(4) Turnell, D. C.; Cooper, J. D. H. Sample Preparation Method for Liquid Chromatography. US 4,837,157, 1989.
(5) Frenzel, W.; Markeviciute, I. Membrane-Based Sample Preparation for Ion Chromatography—Techniques, Instrumental Configurations and Applications. J. Chromatogr. A 2017, 1479, 1–19. DOI: 10.1016/j.chroma.2016.11.052
(6) Frenzel, W. Sample Preparation Techniques for Ion Chromatography - an Overview. In Sample Preparation Techniques for Ion Chromatography; Monograph 8.025.5003; Metrohm AG.
(7) Moldoveanu, S.; David, V. Modern Sample Preparation for Chromatography; Elsevier, 2021.
(8) Soria, A. C.; Brokł, M.; Sanz, M. L.; Martínez-Castro, I. Sample Preparation for the Determination of Carbohydrates in Food and Beverages. In Comprehensive Sampling and Sample Preparation; Analytical Techniques for Scientists; Elsevier, 2012; Vol. 4, pp 213–243.
(9) Suess, E. Simplified Analysis of Dairy Products with Metrohm Inline Dialysis; Metrohm AG, 2023.
(10) Ziegler, C. J.; Suess, E.; Lanciki, A. Improving on AOAC 2001.02: GOS Determination in Foods Using HPAEC–PAD. The Column 2021, 17 (2), 8–13.
(11) Suess, E. Robust Multiparameter Analysis of Infant and Follow-on Formulas with Ion Chromatography (IC), Metrohm AG White Paper, 2022.
(12) Metrohm AG, A Fresh Look at Food and Beverage Analysis, EB-003EN–2022-03, Metrohm AG, 2022.
(13) Liu, X.; Wang, Y.; Cong, H.; Shen, Y.; Yu, B. A Review of the Design of Packing Materials for Ion Chromatography. J. Chromatogr. A 2021, 1653, 462313. DOI: 10.1016/j.chroma.2021.462313
(14) Zatirakha, A. V.; Smolenkov, A. D.; Shpigun, O. A. Preparation and Chromatographic Performance of Polymer-Based Anion Exchangers for Ion Chromatography: A Review. Anal. Chim. Acta. 2016, 904, 33–50. DOI: 10.1016/j.aca.2015.11.012
(15) Gilchrist, E. S.; Healy, D. A.; Morris, V. N.; Glennon, J. D. A Review of Oxyhalide Disinfection By-Products Determination in Water by Ion Chromatography and Ion Chromatography-Mass Spectrometry. Anal. Chim. Acta. 2016, 942, 12–22. DOI: 10.1016/j.aca.2016.09.006
(16) Zhang, F.; Shen, G.; Ji, S.; Yang, B. Recent Advances of Stationary Phases for Hydrophilic Interaction Liquid Chromatography and Ion Chromatography. J. Liq. Chromatogr. Relat. Technol. 2015, 38 (3), 349–352. DOI: 10.1080/10826076.2014.941258
(17) Metrohm AG, Column Manual for Metrosep A Supp 19; 8.0107.8013EN; Metrohm AG.
(18) Weiss, J.; Shpigun, O. Handbook of Ion Chromatography, Vol. 3, 4th ed.; Wiley-VCH, 2016.
(19) Buldini, P. L.; Cavalli, S.; Trifirò, A. State-of-the-Art Ion Chromatographic Determination of Inorganic Ions in Food. J. Chromatogr. A 1997, 789 (1–2), 529–548. DOI: 10.1016/s0021-9673(97)00963-1
(20) Sargen, M.; Utter, D. Biological Roles of Water: Why Is Water Necessary for Life? Blog, Special Edition: Water, uncategorized 2019. https://sitn.hms.harvard.edu/uncategorized/2019/biological-roles-of-water-why-is-water-necessary-for-life/ (accessed 2023-05-24).
(21) WHO, Guidelines for Drinking-Water Quality. World Health Organization, First addendum to 3rd edition, Vol. 1, Recommendations, ISBN 92 4 154696 4 2006.
(22) WHO, A Global Overview of National Regulations and Standards for Drinking-Water Quality, Second Edition; 2021.
(23) Levallois, P.; Villanueva Belmonte, C. Drinking Water Quality and Human Health; MDPI, 2019.
(24) Villanueva, C. M.; Kogevinas, M.; Cordier, S.; et al. Assessing Exposure and Health Consequences of Chemicals in Drinking Water: Current State of Knowledge and Research Needs. Environ. Health Perspect. 2014, 122 (3), 213–221. DOI: 10.1289/ehp.1206229
(25) EPA, Safe Drinking Water Act (SDWA); United States Environmental Agency, 1996.
(26) EPA, Method 300.1: Determination of Inorganic Anions in Drinking Water by Ion Chromatography, Revision 1.0.; United States Environmental Agency, 1997.
(27) Metrohm AG, Quality Control of Dialysis Concentrates. Metrohm Application Note, 2022.
(28) Sun, B.; Wang, J. Food Additives. In Food Safety in China: Science, Technology, Management and Regulation, Jen, J. J.; Chen, J., Eds.; John Wiley & Sons Ltd, 2017.
(29) Nabavi, S. M.; Nabavi, S. F.; Loizzo, M. R.; et al. Food Additives and Human Health; Bentham Science Publishers, 2020.
(30) Inetianbor, J. E.; Yakubu, J. M.; Ezeonu, S. C. Effects of Food Additives and Preservatives on Man - a Review. AJST 2015, 6 (2), 1118–1135.
(31) Abdelghany, T. Safe Food Additives. A Review. J. Biol. Chem. Research 2015, 32 (1), 402–437.
(32) Carocho, M.; Barreiro, M. F.; Morales, P.; Ferreira, I. C. F. R. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13 (4), 377–399. DOI: 10.1111/1541-4337.12065
(33) The European Parliament and the Council of the European Union (2018), Regulation (EU) Regulation (EC) No 1333/2008 of the European Parliament and the Council of 16 December 2008 on Food Additives, Official Journal of the European Union, 31.12.2008, L 354/16-33, https://eur-lex.europa.eu/eli/reg/2008/1333/oj (accessed 2023-05-24).
(34) Joint FAO/WHO Codex Alimentarius Commission (1995), General Standard for Food Additives, CODEX STAN 192-1995, https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B192-1995%252FCXS_192e.pdf (accessed 2023-05-24).
(35) Garcia Fuentes, A.; Röttger-Wirtz, S.; Vos, E.; Verhagen, H. Short Review of Sulphites as Food Additives. European J. Nutr. Food Saf. 2015, 5, 113–120. DOI: 10.9734/EJNFS/2015/11557
(36) Liao, B. S.; Sram, J. C.; Files, D. J. Determination of Free Sulfites (SO3–2) in Dried Fruits Processed with Sulfur Dioxide by Ion Chromatography through Anion Exchange Column and Conductivity Detection. J. AOAC Int. 2013, 96 (5), 1103–1108. DOI: 10.5740/jaoacint.11-053
(37) Food Additives, 2nd ed.; Branen, A., Davidson, P., Salminen, S., III Thorngate, J., Eds.; CRC Press, 2001. DOI: 10.1201/9780367800505
(38) efsa, Sulfites: Safety Concern for High Consumers, but Data Lacking; European Food Safety Authority, 2022.
(39) Zierfels, G. Simplified Sulfite Determination in Foods and Beverages Using Ion Chromatography. Metrohm AG White Paper, 2021.
(40) Espinosa, M.; Lanciki, A. A Simplified Method to Determine Total Sulphite Content in Food and Beverages via Ion Chromatography. The Column 2020, 16 (2), 12–16.
Vincent Diederich is currently a product manager for ion chromatography columns in the Metrohm Competence Center for Ion Chromatography. After completing his M.Sc. in chemical and bioengineering at ETH Zurich, he remained there to acquire a Ph.D. at the Institute for Chemical and Bioengineering (ICB). In 2015, he joined Metrohm AG and has been a member of the IC team since 2018.
Elke Süss is currently an application specialist in the Metrohm Competence Center for Ion Chromatography. With an M.Sc. in geoecology, she received a Ph.D. in environmental geochemistry in 2011 from Bayreuth University, Germany. In 2018, she joined Metrohm AG as a member of the IC team.