- June 2023
- Volume 19
- Issue 6
- Pages: 27–29
Book Review: Practical Application of Supercritical Fluid Chromatography for Pharmaceutical Research and Development
Why is SFC becoming increasingly more important as a sustainable analytical technique?
A review of Practical Application of Supercritical Fluid Chromatography for Pharmaceutical Research and Development and why SFC is becoming increasingly more important as a sustainable analytical technique.
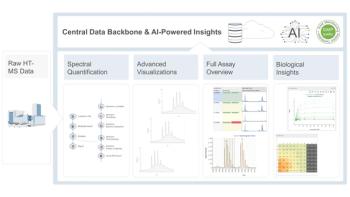
In late 2019 we were approached by Elsevier to submit a proposal for the development of a text on supercritical fluid chromatography (SFC), focusing specifically on pharmaceutical analysis, where this technique has current and potentially untapped applications. As long‑standing users of SFC, we felt it has been underutilized in the pharmaceutical industry, especially in late‑stage analytical development that relies heavily on ultrahigh-pressure liquid chromatography (UHPLC) for many drug substance and product characterization activities. As a reflection of this, when we approached the United States Pharmacopeia (USP) regarding their viewpoints on the technique, we found that SFC was not in the current pharmacopeial compendium of methods, simply due to its very low frequency of use, and therefore general chapter guidance was also unavailable. To address these gaps, we wanted to develop a detailed text that industrial analytical chemists would find helpful in solving real-world pharmaceutical challenges using SFC, but also provide readers new to the technique with sufficient background detail as to “what” the technique is and “where” it can be utilized (Figure 1).
We were fortunate that many of our industrial and academic collaborators shared our interest in bringing this greener and more sustainable separation tool into greater focus. We recognized that SFC has made significant advances in recent years due to more reliable instrumentation, new column chemistries, and increased theoretical understanding—and this text provided a good opportunity to highlight these developments.
Our goal was to ensure chapters progressed through the pharmaceutical development timeline, from drug discovery to commercial manufacture, and were incremental in expanding the readers’ understanding across the text. The thirteen chapters offer a broad range of theoretical and practical information from experts in the field, to provide the reader with the knowledge to develop robust SFC methods. The initial chapters discuss where the technique can be applied and offer fundamental knowledge to understand instrument operational design including column and mobile phase selection. Subsequent chapters provide insight on method development in early discovery compound screening, bioanalysis and preparative isolation, drug substance and intermediates in synthetic process development, and formulated drug product characterization through to commercial methods (Table 1). To this latter point, we supplemented principles and theory with a forward look at how this technique could be helpful for regulatory submissions for methods in support of drug manufacturing activities. Other chapters provide practical insight into detector selection and instrument and method troubleshooting approaches. The concluding chapter ties the entire book together, highlighting key points from every chapter, and offering insight into future development needs and possible scientific avenues. Where appropriate, guidance in the chapters is reinforced with real-world case studies and other relevant insights around validation, instrument and method repeatability, and method transfer.
We feel that SFC is not just a needed tool in the pharmaceutical industry, but a necessary one for advancing sustainable methods across many application areas. While the book’s scope is focused on pharmaceutical analysis, the information provided is relevant to academic teaching and other industrial areas, such as agrochemical, natural product isolation, and neutraceuticals, as well as for rapidly growing industries for medicinal, environmental, and potentially petrochemical researchers. We feel that our book will assist researchers looking to develop more sustainable ways to separate analytes and increase their separation options for their most challenging separations. We hope you agree.
About the Authors
Paul Ferguson is a principal scientist at AstraZeneca specializing in separation science. He has over 20 years’ experience working in major pharma in early- and late-stage development and first started working with SFC in 2006. Michael Hicks is an associate principal scientist at Merck & Co., Inc., Rahway, New Jersey, USA, with over 25 years doing pharmaceutical research, specializing in chromatographic development from early drug discovery through to final product filing.
Articles in this issue
over 2 years ago
Determining Benzalkonium Chloride in Pharmaceutical Formulationsover 2 years ago
June 2023 News in Briefover 2 years ago
Do Soil Samples Contaminate the Gas Chromatography System?over 2 years ago
Rising Stars of Separation Science: Leo Wang Hantaoover 2 years ago
Vol 19 No 6 The Column June 2023 Europe & Asia PDFover 2 years ago
Vol 19 No 6 The Column June 2023 North American PDFNewsletter
Join the global community of analytical scientists who trust LCGC for insights on the latest techniques, trends, and expert solutions in chromatography.