What the New USP Chapter <621> Guidelines Mean for Your HPLC Analysis
The US Pharmacopeia (USP) monographs are designed with top-of-the-range high performance liquid chromatography (HPLC) columns at the time when the method is established. As time progresses and new technologies are brought to market, methods may be eligible for updating as there is a larger focus on method life cycle management from regulators. Technology has significantly advanced since the inception of the USP method, offering optimization opportunities. Now, the US Pharmacopeia allows method improvements to meet new standards, with changes to particle size, length, and inner diameter approved. This article explores what this transition to newer technology looks like, with an example applied to determining organic impurities of pramipexole dihydrochloride.
The US Pharmacopeia (USP) provides a global and extensive source for upholding medicine quality, publishing over 5000 standards for medicines that cover both active and inactive pharmaceutical ingredients. Their monographs describe tests to validate medicines, meeting the criteria and quality expectations required across the pharmaceutical space. One such document, the USP General Chapter <621> “Chromatography”, upholds the rigorous standards required in chromatographic techniques.
Composite image of compass | Image Credit: © WavebreakMediaMicro - stock.adobe.com

Although the USP monographs are robust and effective, they are not perfect. While the tests were originally designed using the highest-quality equipment at the time, as time progresses and technology advances, state-of-the-art status fades, and the methods are no longer fit for modern-day challenges.
Most crucially, environmental impact is a growing consideration for laboratories, with many looking for sustainable approaches to meet their goals. USP chromatographic methods hinder these aspirations, as they often use large volumes of solvents—causing a significant environmental footprint. What’s more, the methods are often slow, limiting experimental throughput. Scientists are desperately seeking to implement newer technologies to make their processes greener, but a lack of approval by the US Pharmacopeia has meant they have been unable to do so—until now.
As of 1 December 2022, the US Pharmacopeia updated General Chapter <621> “Chromatography” to allow changes to columns. Now, the original methods can be improved to new standards (1), enabling laboratories to adopt more sustainable processes. Particle length, size, and internal diameter (i.d.) can be changed, and the variations are valid for both isocratic and gradient methods. The stationary phase, however, must remain the same. Other global organizations have also followed suit to accommodate improved technologies, with the European Pharmacopoeia and the Japanese Pharmacopeia releasing new equivalent editions that were implemented on 1 January 2023 (2). The US regulations, however, remain the most stringent and set the precedent.
While these changes are widely welcome news, it is a challenge to understand how to translate the USP methods to improved versions that can be set up in the laboratory. Proper interpretation and implementation require time investment, in turn increasing costs. While new tools are available to help easily modernize methods (3), a solid understanding of the changes can set a valuable foundation for any modifications.
This article will break down what the changes mean, helping to guide the reader from the original USP methods to the improved versions. To demonstrate the changes, it is shown how they can be applied to the USP standard operating procedure for determining organic impurities of pramipexole dihydrochloride (4), leading to substantial solvent reduction and faster run times.
Materials and Methods
Materials
Potassium dihydrogen phosphate (7778-77-0), sodium-1-octanesulfonate monohydrate (207596-29-0), and phosphoric acid (7664-38-2) were all obtained from Sigma Aldrich. Acetonitrile (75-05-8) was obtained from Fisher Chemicals. Pramipexole for system suitability was obtained from the European Directorate for the Quality of Medicines & HealthCare (EDQM). Screw top vials from Thermo Fisher Scientific were used.
HPLC Setup
HPLC Solvent Setup
The mobile phases, diluent, and system suitability solution were made up as follows:
- Mobile phase A: Potassium dihydrogen phosphate (9.1 g) and sodium 1-octanesulfonate (5 g) were dissolved in water (1 L). Phosphoric acid was used to adjust the pH of the solution to 3.0.
- Mobile phase B: 50:50 acetonitrile and mobile phase A
- Diluent: 20:80 acetonitrile and mobile phase A
- System suitability solution: Pramipexole for system suitability (7.5 mg) was dissolved in the diluent solution (5 mL).
HPLC Column Setup
A 4.6 mm × 150 mm, 5-μm Thermo Scientific BetaSil C18 column was used as a starting point for the original USP method. To investigate the particle size, two columns were tested: a 2.1 mm × 100 mm, 3-μm Thermo Scientific Hypersil Gold C18, and a 2.1 mm × 50 mm, 1.9-μm Thermo Scientific Hypersil Gold.
Experimental Run Conditions
The original method run time was 20 min, with the retention time of pramipexole averaging at 6.74 min. We used an injection volume of 5 μL, a UV detector with 264 nm wavelength, and a column temperature of 40 °C. The original method running conditions were: time (% of B): 0 min (40%), 15 min (80%), 15.1 min (40%), and 20 min (40%).
Results and Discussion
The original pramipexole dihydrochloride method used a standard L1 column with a C18 stationary phase, 5 μm particle size, 4.6 mm i.d., and 150 mm length. Additionally, the suggested flow rate was 1.5 mL/min. The key method criteria are <2.0 for asymmetry of the main peak, and >6.0 for resolution between the main peak and compound A. The allowed revisions to the column method are as shown in Table 1.
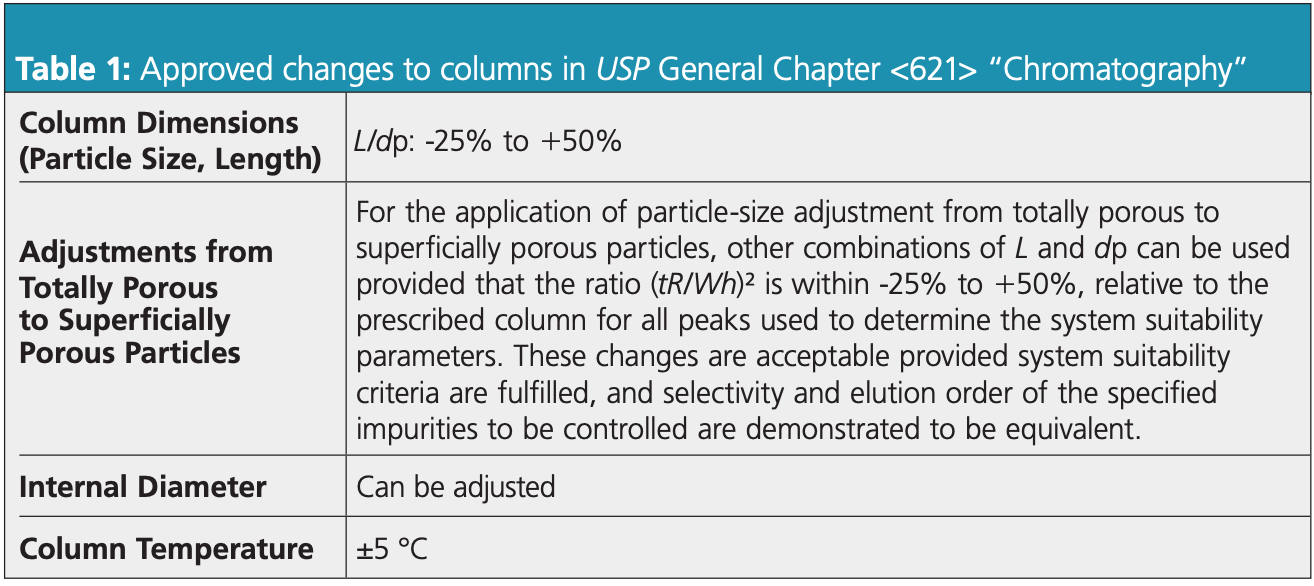
Here, we explore how to modernize your method by changing the particle size, length, and internal diameter.
Particle Size and Length
The USP Chapter <621> updates state that L/dp must be maintained within -25% and +50%, meaning that the percentage difference must be calculated.
A 3 μm column with a length of 100 mm and an internal diameter of 2.1 mm, for example, gives a ratio of 33.3—the original column used has a ratio of 30. The % deviation, outlined in equation 1, demonstrates that the new column is within the approved limits:
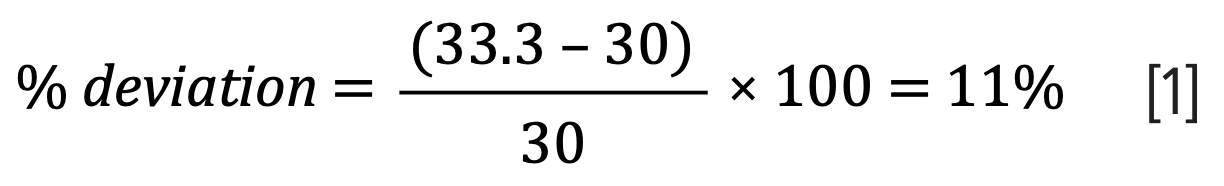
Internal Diameter
When changing the particle size and internal diameter of the column, it is essential to adjust the flow rate accordingly (equation 2):
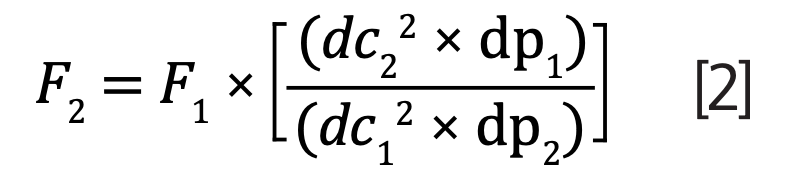
The original USP column possesses the following:
- Flow rate (F1) = 1.5 mL/min
- Internal diameter (dc1) = 4.6 mm i.d.
- Particle size (dp1) = 5 μm
In comparison, the modernized 3 μm column has:
- Internal diameter (dc2) = 2.1 mm i.d.
- Particle size (dp2) = 3 μm
Using equation 2, the new flow rate of the modernized column is 0.52 mL/minute, a reduction of almost a third (equation 3):
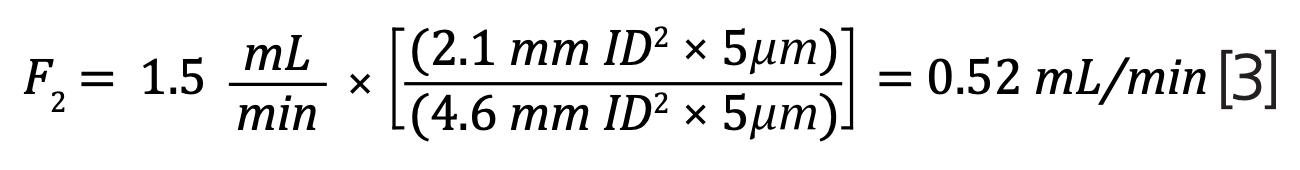
Optimizing Method Conditions
Once the particle size, length, and internal diameter have been considered, the gradient and injection volume also need adjusting to the new method.
For the gradient, the separation should be maintained by keeping the same gradient steps but by calculating new times. The original gradient time, tG1, should be set to 1, and the new gradient time, tG2, can be calculated using equation 4. As shown, the modernized 3 μm column has a gradient time of 0.4 min:
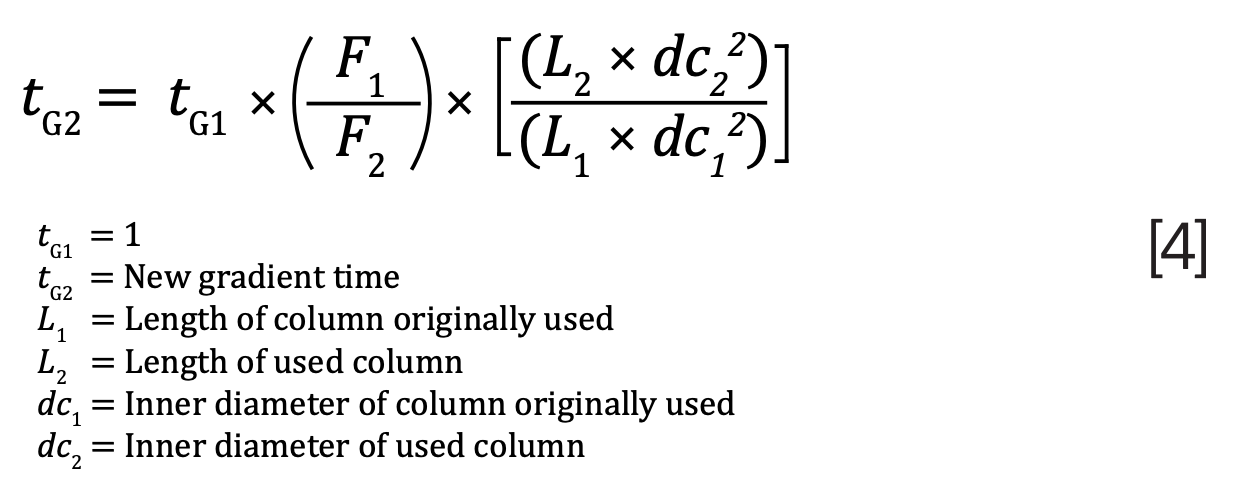
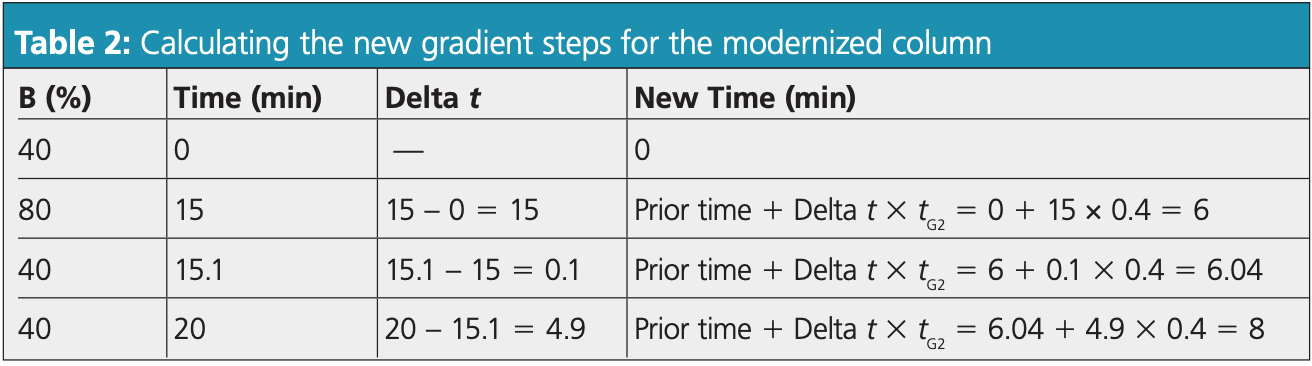
To calculate the new times, tG2 must be multiplied with the delta time for each step and the time of the prior step added. Then, to get the delta time, the current time must be subtracted from the prior time. The new times for pramipexole dihydrochloride using the modernized column are demonstrated in Table 2. Notably, the run time using the modernized column can be drastically reduced from the original 20 min to just 8 min.
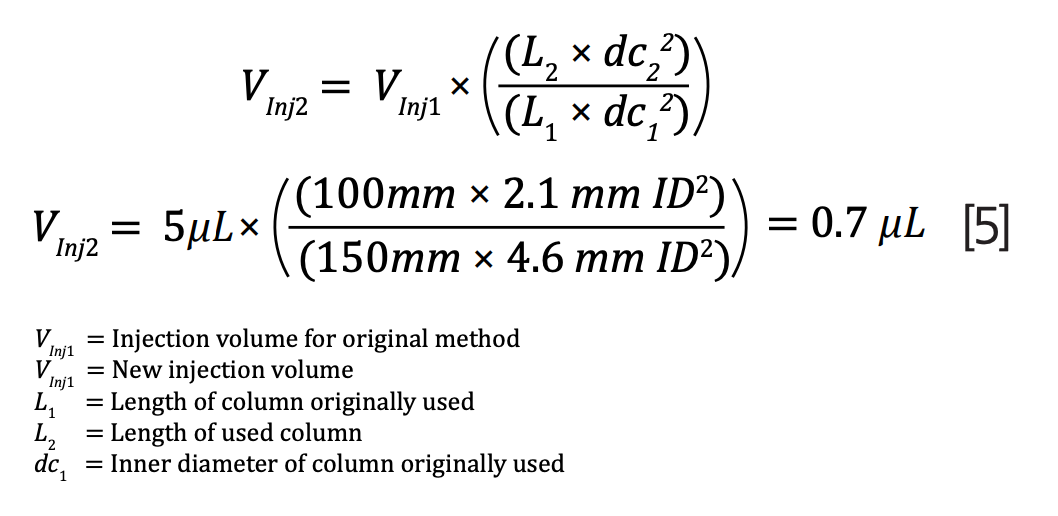
The US Pharmacopeia states that injection volume can also be adjusted, but this is optional. It was not adjusted in this study but can be changed using equation 5:
Modernized USP Optimization Results
The updated method was used to compare two different columns alongside one that matched the original USP particle size of 5 μm. The first, a 3 μm column, was used in the example calculations above. Similar calculations were performed for the second, a 1.9 μm column.
Overall, it was found that modernizing to a 3 μm column could save 60% of the analysis time compared to the original USP standard—offering a retention time of 2.34 min compared to the original 6.72 min (Figure 1). By optimizing to a 1.9 μm column, 87% of the analysis time was saved, owing to a retention time of 0.95 min.
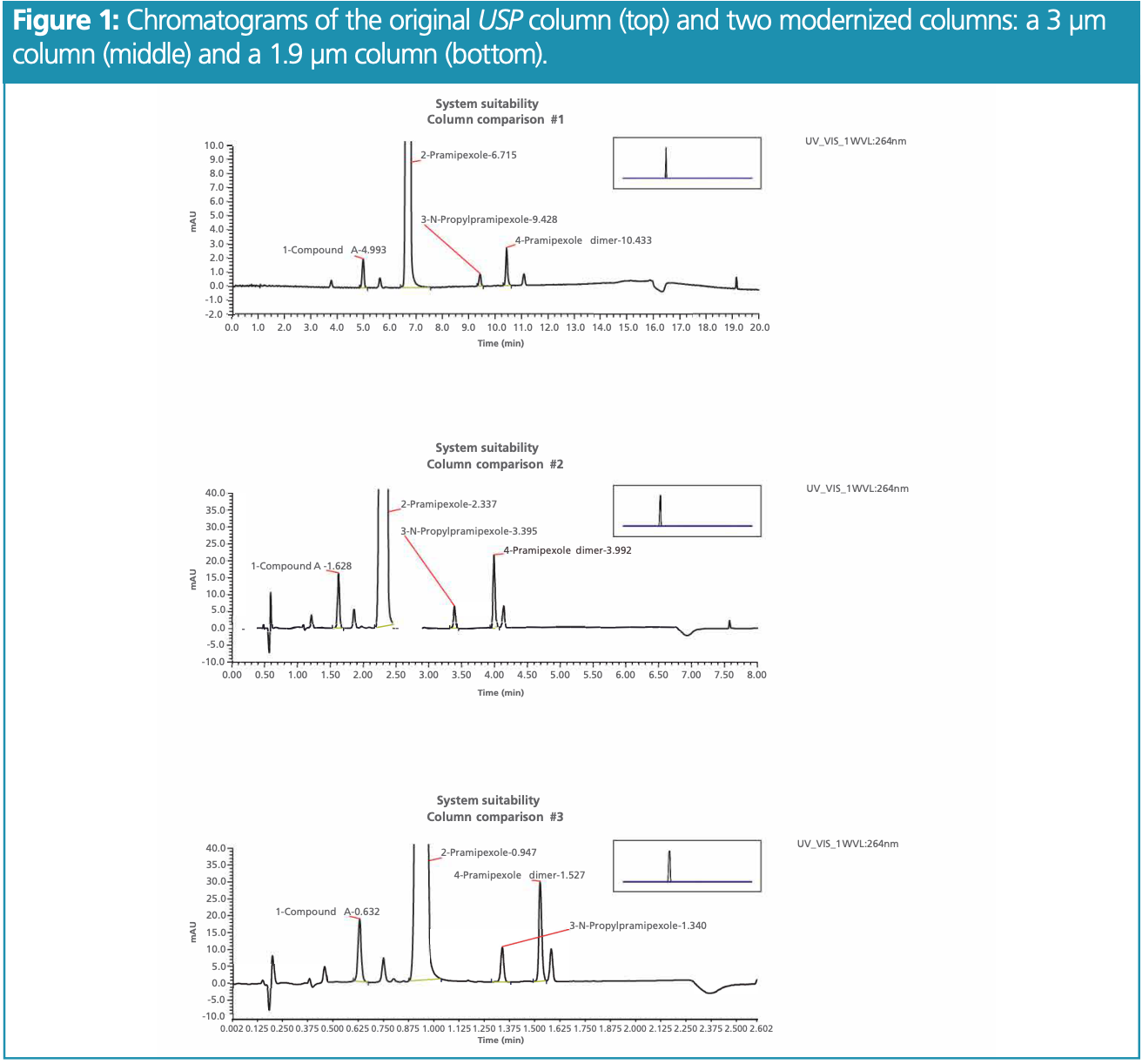
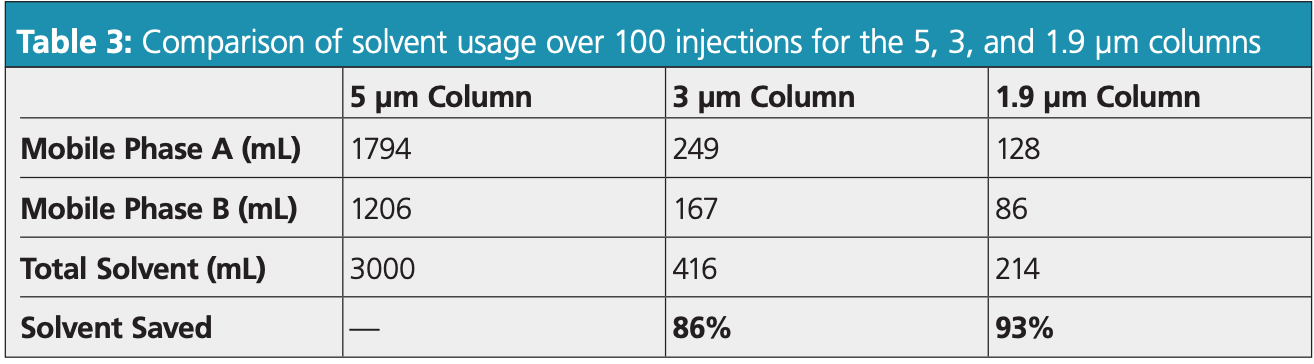
As environmental impact is a key factor for modernization, solvent consumption was also investigated. Lower flow rates and shorter run times led to lower quantities of solvent being required, and the volumes needed for 100 injections were calculated (Table 3). Modernizing the procedure with a 3 μm column or 1.9 μm column slashed solvent consumption by 86% and 93%, respectively.
Conclusion
Modernizing chromatography processes in line with new US Pharmacopeia guidelines is critical to make workflows more environmentally friendly, while cutting down method times and laboratory costs. When choosing a column, there are three steps that you must take to stay within guidelines:
- Determine that the L/dp ratio meets the guidelines;
- Adjust the flow for the new internal diameter;
- Calculate the new gradients and injection volumes.
To illustrate the strengths that a modernized column can bring to the laboratory, changes to the USP monograph for determining organic impurities of pramipexole dihydrochloride were investigated by comparing how a 3 μm column and 1.9 μm column perform against the original 5 μm column. The results were astounding: a 1.9 μm column reduced analysis time by 87% and solvent consumption by 93% compared to the original method. With modernization enabling greener methods and streamlining laboratory analysis, scientists can deliver safer products faster, with a lower environmental footprint than ever before.
References
(1) USP, USP General Chapter <621>, “Chromatography”. https://doi.org/10.31003/USPNF_M99380_06_01 (accessed 2023-05-01).
(2) EDQM, Ph. Eur. Commission adopts harmonised general chapter 2.2.46. Chromatographic separation techniques. https://www.edqm.eu/en/-/ph.-eur.-commission-adopts-harmonised-general-chapter-2.2.46.-chromatographic-separation-techniques (accessed 2023-05-01).
(3) HPLC Column Selection Guide. https://www.thermofisher.com/order/lc-column-guide/ (accessed 2023-02-23).
(4) Gleerup, K.; Kmieliauskaitè, S.; Bilgesoy, S. New changes to the US Pharmacopeia: Reduce time and solvent usage with newer technology columns. https://assets.thermofisher.com/TFS-Assets/CMD/Reference-Materials/wp-001210-ccs-hplc-columns-hypersil-usp-method-wp001210-na-en.pdf (accessed 2023-05-01).
About the Author
Kaja Gleerup is a product marketing manager at Thermo Fisher Scientific. In her role, Kaja supports scientists in method development and optimization using her extensive technical knowledge gained over 15 years. Kaja is experienced in good manufacturing practice (GMP) for protein therapeutics, and aids with the theory and applications of both specific and groups of analytes at multiple manufacturers.
E-mail: kaja.gleerup@thermofisher.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.