The Early Days of HPLC at DuPont
LCGC North America
Guest columnist Dick Henry traces the history of HPLC back more than 40 years, starting with his days at DuPont.
When one is approached to write an article about the history of high performance liquid chromatography (HPLC), it is both a compliment and a vivid reminder that you have been around a long time. One of the first things that I recalled was a project that started about 10 years ago with Dave Carr and Mike Henry, who have been well known HPLC contributors over the years. In 1997, Dave and Mike decided to preserve some history by interviewing as many chromatographers as possible who were around during the early days of HPLC. Their plan was to publish a book about the interesting history of HPLC before too much first-hand information was lost. The book was never published, but I felt that some of the material gathered by Dave and Mike might be of interest to the many readers of such a widely circulated magazine as LCGC. Dave Carr dug into his archives and was able to locate a first-person account supplied by Dr. Jack Kirkland (1) about the early days in HPLC from his perspective. Those days corresponded to my own, so I was pleased to find a record for comparison. To keep my dates consistent, I also referred to an article about Jack authored by Klaus Unger (2) as I wrote this historical account from my own memory and perspective.
When modern HPLC was emerging, I was just beginning my own professional career as a new DuPont employee at the Experimental Station in Wilmington, Delaware, where Jack worked. I graduated from Penn State University (University Park, Pennsylvania) in 1966, where I did thermal analysis under Professor Joseph Jordan and shared laboratories with Peter Carr, who also later converted to HPLC and made important contributions to its development. I conducted my first HPLC experiments as a postdoctoral student in 1966–1967 at Purdue University (West Lafayette, Indiana) with Professor Lockhart Burgess (Buck) Rogers, who was always one of the first people to recognize the importance of a new analytical technique. Interestingly, I shared labs at Purdue with Ron Majors and Brian Bidlingmeyer, who were graduate students in liquid separations. The fact that I have remained involved with HPLC from its beginning is due in large part to the many cordial relationships that I have enjoyed over the years. My HPLC career can be divided into three parts: the early years at Dupont, the middle years in California, when I traveled extensively and met many scientists in Europe and Japan, and the later years when I returned to Pennsylvania to teach at Penn State and start Keystone Scientific. This article will focus on the early years. Perhaps I will have a chance to write more about the others in the future.
The DuPont Years
I arrived at the DuPont Experimental Station in 1967 as a research chemist at Carothers Lab. It was named after Wallace Carothers, who invented the first true synthetic fiber, nylon, in 1931. I immediately joined the Analytical Discussion Group that met regularly in the Experimental Station cafeteria to share exciting analytical results. One of the first lunch talks was given by Jack Kirkland, who was already a respected analytical chemist within DuPont, as well as an expert in the still relatively new technique of gas chromatography (GC). Because he worked in the Biochemicals Department, there were many problems that could not be solved by GC because of the thermal instability of the solutes. In 1964, Jack visited the laboratories of Josef Huber in Europe, where important HPLC research was being conducted, and he recognized the value of this technique for his work back at DuPont. Jack became convinced that better particles would unlock the LC performance predicted in the early 1960s by Cal Giddings, John Knox, Josef Huber, and other scientists, so he returned to the Experimental Station and immediately gained approval to conduct research aimed at creating improved particles for HPLC. His stimulating talk that day was about a new superficially porous particle; it was named Zipax, which rolls off the tongue much more easily than superficially porous. Zipax was invented in collaboration with Ralph Iler, a DuPont scientist who many considered to be the father of silica and silicone chemistry (3). Zipax spheres had a particle size of 30–40 μm and showed higher efficiency than other available HPLC particles due to their uniform size and a thin porous diffusion layer, but they were hampered by low surface area that could support only about 1% stationary phase (w/w). After hearing Jack's lunch talk, I became very excited by the promising new HPLC technology and wanted to become part of it, even though I knew that a satisfying career like Jack's in analytical separation would be very hard to achieve at Carothers Lab. I was further inspired at that time by a visit to Paul Hamilton at nearby A. I. DuPont Institute (Wilmington, Delaware), where Paul was achieving very impressive amino acid separations on small particle ion-exchange resins (4). By 1969, I had transferred from Carothers Lab to become one of the early employees of the newly formed DuPont Instrument Products Division (IPD) located just a few miles south of the Experimental Station in Glasgow, Delaware. Before leaving DuPont in 1973, I continued to work closely with Jack at the Experimental Station along with other scientists at IPD, including Joe DeStefano, John Larmann, Bob Moody, and others. This group of friends later formed Rockland Technologies and continued to build upon the success of another product developed by Jack, the small, totally porous Zorbax HPLC particles.

Figure 1
When I arrived at IPD, I vividly remember finding a nearly empty HPLC laboratory except for three things: a prototype HPLC instrument called the Model 820 (Figure 1), a large box full of customer samples, and a bottle of Zipax particles to coat with liquid phase. At that time, we used primarily β, β'-oxydipropionitrile (abbreviated ODPN or BOP for obvious reasons) as the liquid stationary phase; the ODPN-coated Zipax was tap-packed into straight 50 cm × 2.1 mm columns. Injection was made with GC syringes though a septum that lasted for only a few hours once pierced, even though septa were made with the best DuPont fluorocarbon elastomer technology. The Model 820 HPLC instrument was quite innovative at the time because it featured a 3000 psi pneumatic pump that had a higher pressure rating than most other HPLC instruments available in the early 1970s. Gas amplifier pumps operate at constant pressure and were an excellent choice because they did not require leak-free seals and check valves for steady flow and good quantitation. Our choice of a pneumatic pump was even more important because a leaking septum during the run did not destroy the integrity of the analytical data for that sample; flow would hold very constant, even with various small system leaks, because column inlet pressure was controlled. The biggest disadvantage of constant-pressure HPLC instruments was that flow diminished and peak areas increased when the column became even slightly plugged; retention time had to be monitored carefully to know when this was happening. As in GC, use of internal standards was important for accurate quantitation. Pneumatic pumps are still important in HPLC, although more convenient, electromechanical metering pumps have replaced them in most, but not all, HPLC instruments. Hardware milestones achieved at DuPont IPD in the early 1970s included the development of a unique, low-dwell-volume gradient device that blended digitally and accurately at high pressure after the pump. Another significant hardware development at IPD was the replacement of the septum injector with a loop injection valve. In the early 1970s, Stan Stearns of Valco (then headquartered in Houston, Texas) visited our IPD laboratory, where we successfully tested a GC valve that had been modified to operate at higher pressure for HPLC; Stan and I have continued to maintain a productive relationship over the years.
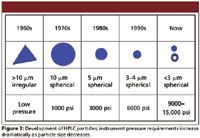
Figure 2
Unfortunately, columns with a liquid phase coating usually lasted only about a day or two before losing too much retention as the immiscible (not insoluble) liquid phase gradually moved from column to waste reservoir. Gradients were impossible with these early "stationary" phases, so polymer coatings were developed that improved column stability and even allowed gradient elution. Three important polymer phases, Zipax SCX, Zipax SAX, and Zipax HCP, emerged from DuPont's exceptional polymer technology and became very popular in the 1970s. The first success, Zipax SCX, was the subject of Kirkland U.S. Patent 3,577,266, issued on May 4, 1971. It used a coating of Nafion or sulfonated tetrafluorethylene copolymer discovered in the late 1960s by Walther Grot of DuPont; it had important HPLC applications for herbicides and other cationic solutes. Zipax SCX would have no doubt become popular in the pharmaceutical industry, but HPLC had not yet been accepted by the FDA. Zipax SAX was made from a quaternized hydrophobic polymer that was an ingredient in hair spray designed to preserve the rigid hair styles of the times; Zipax SAX was excellent for separation of anionic solutes and became one of the first HPLC packings accepted by the FDA for routine analysis of food dyes. Zipax HCP, an abbreviation for hydrocarbon polymer, was one of the very first commercial phases to employ reversed-phase conditions. It was made from a rubber-like, ethylene–propylene copolymer designed for garden hoses and other premium applications in which stability and flexibility over a range of temperatures was needed; Zipax HCP was an excellent general purpose phase for all hydrophobic solutes and quickly became the preferred column in my toolbox, much like C18 phases are today. During this same time period, Jack also developed and patented one of the first highly stable bonded phases based upon silicone chemistry. Called Zipax Permaphase, it made our life in the laboratory much easier, especially when coupled with gradient elution and an injection valve. Permaphase ODS (C18) and ETH (Ether) demonstrated that stable covalent bonding would be the direction for all future HPLC particles; however, we continued to solve numerous problems with polymer-coated Zipax while we developed improved silica particles and additional bonded phases.
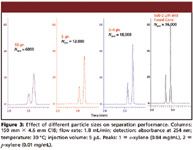
Figure 3
Although Zipax and other superficially porous particles (some were then called pellicular), such as Corasil I and II from Waters (Milford, Massachusetts) and Perisorb from E. Merck (Darmstadt, Germany), were important stages in the evolution of HPLC in the late 1960s and early 1970s, the surface areas were too low (1–10 m2/g); solute retention was barely adequate for many solutes. Small porous spheres were being developed that had much higher surface areas. Just before I left DuPont in 1973, I had the chance to work with Zorbax, another one of Jack's creations that used Ralph Iler's silica–polymer aggregation (coacervation) technology during synthesis. The first Zorbax particles were 7 μm in size and had 60-Å pores with a surface area of 300 m2/g. Although Zorbax was the first commercially available porous spherical particle to my knowledge, porous spherical particles such as Spherisorb, Hypersil, and others also were being developed in Europe. Long columns filled with superficially porous particles were supplanted quickly by shorter, more retentive columns filled with high-efficiency, porous silica.
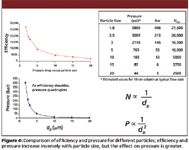
Figure 4
Trends in HPLC Particle Technology
The history of HPLC is primarily about the history and evolution of particle technology. While instrumental developments such as improved pumps, gradient formers, valves, and detectors have all been important, most of the real innovations that have propelled the HPLC technique to prominence have been related to HPLC particles and columns. Figure 2 depicts the history of HPLC particle technology, in which important new developments have occurred on a regular basis for more than 40 years. While porous layer particles made a brief appearance in the 1960s and early 1970s, the steady trend has been to reduce particle size to improve efficiency, as shown in Figure 3. Better column efficiency slows the rate of band spreading and creates narrower peaks, allowing columns to deliver higher resolution and peak capacity. More efficiency also enables more speed and less solvent consumption because shorter columns can give the same separation as longer, larger particle columns. Efficiency increases inversely with particle diameter, as shown in Figure 4, and resolution only increases with the square root of efficiency. Because pressure drop increases inversely with the square of particle diameter, the cost of increasing resolution using smaller particles is very high. Every time particle size is reduced significantly, another round of instrument development usually must occur to handle the pressure. In 1965, Cal Giddings wrote about the effect of mean particle diameter on HPLC column performance (5), "The foregoing arguments indicate that a zero particle diameter is the optimum. However, very little resolution gain is found beyond a certain reduction in dp because the term mainly affected, ωdp2v/Dm, rapidly becomes neglible. Thus, a point is reached in reducing dp where there is very little theoretical advantage, and severe practical disadvantage to further reduction. The practical disadvantages stem from the excessive pressure drop needed to force mobile fluid through the column and the difficulty of preparing a uniform packing of extremely fine materials." Cal was already explaining that the C-term alone would not be able to deliver better small molecule performance below a certain particle size and was pointing to the A-term or flow uniformity for additional gains.

Figure 5
Jack Kirkland's latest HPLC particles (6–8) might be his most impressive accomplishment because he has been able to achieve almost twice the efficiency of 3-μm particles without a significant increase in operating pressure. Figure 5 shows an electron micrograph of cross-sectioned 1.7-μm solid-silica spheres that have been coated with a 0.5-μm porous layer. The 2.7-μm porous-layer particle retains about 75% of the surface area and sample capacity of a porous particle. The most remarkable attribute of this particle is that it shows comparable efficiency to a much smaller porous particle in the 1.7–1.8 μm range, as shown in Figure 6. While higher efficiency with porous-layer particles has been clearly demonstrated for larger molecules that benefit greatly from reducing the path of diffusion into the particle (that is, reducing the C-term of the van Deemter equation), the extremely high efficiency of the new particles is surprising for small molecules. The theoretical explanation for the remarkable performance of these denser particles can lie partly in their very narrow particle size distribution, which in turn greatly improves flow uniformity along the column (reducing the A-term of the van Deemter). Called Fused-Core, the new porous-layer particles are produced by Advanced Materials Technology (Wilmington, Delaware) and are offered commericially by Mac-Mod Analytical (Chadds Ford, Pennsylvania) under the tradename Halo and by Supelco Division of Sigma-Aldrich (Bellefonte, Pennsylvania) under the tradename Express. I am happy to be associated with Jack again in my capacity as a consultant for Supelco.

Figure 6
Richard A. Henry, Consultant
References
(1) D. Carr, private communication.
(2) K. Unger, J.Chromatogr., A 1060, 1–7 (2004).
(3) R.K. Iler, The Chemistry of Silica, Edition 1 (John Wiley & Sons, Incorporated, New York, 1979).
(4) P.B. Hamilton, Anal. Chem. 32(13), 1779 1781 (1960).
(5) J. Calvin Giddings, Dynamics of Chromatography, Part I. Principles and Theory (Marcel Dekker, Inc., New York, 1965), p. 281.
(6) J.J. DeStefano, T.J. Langlois, and J.J. Kirkland, J. Chrom. Sci. 46, 254–260 (2008).
(7) J. Kirkland, private communication.
(8) J. DeStefano, private communication.
Acknowledgment
I would like to thank Ron Majors for asking me to write this article and also thank Supelco Division of Sigma-Aldrich and Advanced Materials Technology for supplying certain Figures.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.













