A Mass Spectrometry Primer, Part IV
LCGC North America
This column completes the multipart MS primer with a list of terms derived from common usage throughout various industries.
This issue completes the multi-part MS primer with a list of terms derived from common usage throughout various industries and, while included with the MS Primer. is intended to stand alone. A number of current books recognize the need to monitor and modify the language we use within LC–MS. When a practice such as LC–MS becomes so diverse and prolific the terms and phrases adopted from various sources finding a way into the practice need scrutiny. Many books such as the "Mass Spectrometry Desk reference" (2nd edition, O. David Sparkman, paperback 198 pages published by Global View Publishing, June 30, 2006) provide more than a description of fundamentals making an attempt to explain and unify usage.

Michael P. Balogh
Although I prefer IMMS there may be little problem in most cases determining that 'IMS' means ion mobility mass spectrometry and not imaging mass spectrometry let alone standing as a reference to Dr. Jed Diamond's irritable male syndrome (although I'm sure cases could be made).
My thanks to everyone – I look forward to the comments posted in the margin at www.Waters.com (Resource Library/Primers). The web version and the hardcopy (due out in early 2009) have clearly benefit from your contributions.
Glossary
The following list of terms is derived from common usage throughout the industry as an adjunct to the discussions in this primer and includes terms and techniques no longer in common usage.
Abundance: When viewed as similar to absorbance displayed on a UV detector, the vertical increase in signal above background indicates an increased occurrence of that particular ion (when the x axis is calibrated in mass units) or total ions present (when the horizontal axis is calibrated in time or scans). The signal for all ions resulting from the fragmentation of a single analyte or compounds compared to a base peak (the relative abundance of each ion) is used to determine the fit of a fragmented pattern to a library spectrum for positive identification.
Accurate mass: The measured mass value for a compound with an associated error like 5 ppm. Accurate mass also is used commonly to refer to the technique rather than the measured mass. Exact mass is the exact theoretical value for the mass of a compound.
Atmospheric solids analysis probe (ASAP): Based upon work by Horning in the 1970s, this form of sample ionization developed by McEwen and McKay uses a standard atmospheric pressure chemical ionization (APCI) plasma but forms ions by placing the sample in a heated nitrogen stream. The heat volatilizes a surprisingly large number of samples, and ions are formed by charge exchange with metastable ions created by the APCI plasma. Relatively unambiguous identifications can be made of individual compounds from complex mixtures at low levels using accurate mass instruments. See also DART and DESI.
Atmospheric pressure ionization (API): The term used to refer generally to techniques such as electrospray ionization (ESI) and APCI and others that operate at atmospheric pressure.
Atmospheric pressure chemical ionization (APCI): Originally called solvent-mediated electrospray, it is applied successfully more often to neutral molecules that do not ionize easily directly out of solution. APCI provides a current on a sharp pin, positioned in the on-coming aerosol stream, to create a plasma of metastable ions from the solvent itself and transfer the charge from these ions to the analyte as it passes through the plasma. Heating a probe through which the liquid chromatography (LC) or solvent stream passes creates the aerosol.
Atmospheric gas chromatography: Developed by Charles McEwen at DuPont in 2002. Using a heated transfer line, a standard gas chromatography (GC) effluent can be introduced to a standard API (or ESI–APCI) source on a mass spectrometer. This provides an easy and fast changeover from ESI to gas chromatography (GC) for compounds that would be best analyzed by GC. Mode of ionization can be either APCI or atmospheric pressure photoionization (APPI).
Atmospheric pressure photoionization (APPI): Developed in the 1980s but commercialized after 2000 when krypton gas lamps were found to generate sufficient photon energy at 10 eV (approximately) to ionize nonpolar analytes such as PAHs and steroids not typically amenable to ESI and APCI ionization.
Base peak: Usually the most intense peak in the spectrum to which others are compared; in ionization techniques that give extensive structural information such as electron ionization (EI), the base peak may not be the parent or molecular ion.
Calibration: Substances of known mass are introduced usually as a constant flowing stream while the mass spectrometer software acquires a signal for a given set of filtering conditions (that is, RF/DC ratio for a quadrupole instrument). After comparing the acquired signal to a reference file, a calibration look-up table is created in the software. The calibration table is then the basis for the mass-to-charge ratios passed by the quads to be assigned a specific value. See the MS Primer sections on "Quantitation and Calibration."
Charge residue mechanism: Related to electrospray ionization; a theory first proposed in 1968 by Malcolm Dole in which he hypothesized that as a droplet evaporates, its charge remains unchanged. The droplet's surface tension, ultimately unable to oppose the repulsive forces from the imposed charge, explodes into many smaller droplets. These Coulombic fissions occur until droplets containing a single analyte ion remain. As the solvent evaporates from the last droplet in the reduction series, a gas-phase ion forms.
Chemical ionization (CI): Collisions induced at low vacuum (0.4 torr) by the introduction of a reagent for the purpose of enhancing the production of molecular ions and often sensitivity; as this is a much lower energy process than electron impact ionization, fragmentation is reduced and it is often referred to as a soft ionization technique. See electron ionization.
Collision-induced dissociation (CID): Also referred to as collisionally activated dissociation (CAD), it is a mechanism by which molecular ions are fragmented in the gas phase by acceleration (using electrical potential) to a high kinetic energy in the vacuum region followed by collision with neutral gas molecules such as helium, nitrogen or argon. A portion of the kinetic energy is converted or internalized by the collision, which results in chemical bonds breaking and the molecular ion is reduced to smaller fragments. Some similar "special purpose" fragmentation methods include electron transfer dissociation (ETD) and electron-capture dissociation (ECD). See the MS Primer section on "Biomolecular ionization methods."
Direct analysis real time (DART): Developed by Robert Cody and others in 2002, similar to desorption electrospray ionization (DESI) in application although more closely related to APCI in function. A sample is placed on a substrate and bombarded by energized particles formed in a process similar to APCI. That is, metastable ions are formed by a plasma and transported by heated nitrogen gas directed at the target. See also McEwen's work listed under atmospheric GC and ASAP.
Delayed extraction (DE): Developed for matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) instruments, it "cools" and focuses the ions for approximately 150 ns after they form and before accelerating the ions into the flight tube. The cooled ions have a lower kinetic-energy distribution than uncooled ones, and they ultimately reduce the temporal spread of the ions as they enter the TOF analyzer, resulting in increased resolution and accuracy. DE is significantly less advantageous with macromolecules (for instance proteins >30,000 Da).
Desorption electrospray ionization (DESI): First described by Graham Cooks in 2002 as a means of producing soft secondary ions from (typically) an inert substrate surface. Analogous to MALDI using an ESI probe aimed at about 50° incident angle to the surface allowing ions to chemically sputter and be admitted to the mass spectrometer. Shown to produce information directly from many polar and nonpolar surface materials (skin, intact fruit for pesticide residue detection, and so forth) without the need for sample preparation. See also McEwen's work listed under atmospheric GC and ASAP.
Desorption ionization on silica (DIOS): Once viewed as an alternative to a preparing samples in a MALDI substrate, especially for small molecules because the substrate (a bare silica surface) would not generate interfering ions. Its commercial potential in the late 1990s diminished as the difficulties of producing the plates and the surface susceptibility to contamination became apparent.
Direct current (DC): For our interests, the term usually is used in conjunction with "radio frequency" (RF) when describing how the quadrupole functions as a mass filter. Superimposed RF and constant DC potentials between four parallel rods were shown by Wolfgang Paul in 1953 to act as a mass separator, or filter, where only ions within a particular mass range, exhibiting oscillations of constant amplitude, could collect at the analyzer.
Electron ionization (EI): Sometimes incorrectly referred to as "electron impact" ionization resulting from the interaction of an electron with a particle (atom or molecule); can be thought of as a "hard" ionization technique, as sufficient energy is imparted to disrupt internal chemical bonds requiring high kcal/mol. Ionizing voltage (typically 70 eV) refers to the difference in voltage causing acceleration of the electrons used to induce electron ionization. Unlike CI, EI avoids uncontrolled collisions by operating at high vacuum. The analyzer operates at even higher vacuum (10–4 to 10–6 torr).
Electrospray ionization (ESI): A so-called "soft" ionization technique. The most widely employed of the API techniques. Commercially significant since the late 1980s, the phenomenon is attributed to an excess of energy (voltages in the 3–5 kV range) applied to a conductive tube (stainless steel capillary) inducing the liquid flowing inside to exceed its Rayleigh limits and forming an aerosol upon exiting the tube. The resulting spray (the result of a coulombic explosion) then gives rise to ions contained in the aerosol droplets as they desolvate to approximately a 10-μm radius. The ions typically are protonated and detected in the form M+H in positive ionization mode or M-H in negative ion mode.
Elemental analysis: The nominal mass of an ion, molecule, or radical is the sum of the nominal masses of the elements in its elemental composition. Achieving accurate mass measurement is based upon the calculated elemental composition but the term "elemental analysis" is performed typically on inorganic materials — to determine elemental makeup, not structure — in some cases using solid metal samples. Inductively coupled plasma (ICP) sources are common where a discharge (or lower power-glow discharge) device ionizes the sample. Detection using dedicated instruments, at the parts-per-trillion level, is not uncommon.
Exact mass: The exact theoretical value for the mass of a compound. "Accurate mass" is the measured mass value for a compound with an associated error like 5 ppm. Accurate mass also is used commonly to refer to the technique rather than the measured mass.
Fast atom bombardment (FAB): One of the earlier so-called soft ionization techniques where the result is usually an intense molecular ion with little fragmentation. The analyte is placed in a matrix (often glycerol) either flowing or more commonly placed on the tip of a probe and positioned in the path of high-energy atoms — often xenon or cesium iodide. The technique has been effective for biomolecules up to 10,000 amu, but perhaps more importantly, in conjunction with the magnetic sector mass spectrometer, where exact weight can be determined such as for novel peptides. Sensitivity can be very good (low femtomole levels). The technique can be difficult to master, as the glycerol does foul the mass spectrometer source and the lower masses can be obscured by the presence of glycerol ions. This technique is little used now since the introduction of ESI.
Field ionization (FI): The soft ionization provided by FI leads to little or no fragmentation for a wide variety of analytes. This is particularly significant in petrochemical applications, in which there are limitations to the utility of other ionization techniques due to fragmentation (EI) or complex ionization characteristics (CI). A thin wire with a high applied voltage is heated in the vapor of an organic compound such as indene. The resulting structures that are deposited on the surface of the wire, dendrites, are pyrolized, producing very fine conductive filaments. When a very fine point has a high potential applied to it, a very intense electric field is generated at this tip, providing conditions under which field ionization can take place.
Sample molecules pass in close proximity to the tips of a mass of carbon dendrites grown on the FI emitter. The FI emitter is positioned in close proximity to a pair of hollow extraction rods. The emitter is held at ground potential and a relatively high voltage (12 kV) is applied to the rods, producing very high electric fields around the tips of the carbon dendrites. The GC column is positioned in close proximity and in line with the emitter wire. Under the influence of the electric fields, quantum tunneling of a valence electron from the molecule takes place to give an ion radical.
Flow injection analysis (FIA): This is the practice of introducing a sample (usually purified in an earlier step, as a fraction to remove interferences and complexity from the resulting spectrum) through the LC injector, but without a column in-line. The LC system acts as a sample introduction device only.
Filament: In EI the filament is the source of electrons that interact with the analyte to ionize it. Typically made from a metal wire (flat or round) capable of giving off 70-eV electrons as it heats from current passing through it.
Fragment ion: An ion produced as the loss from a parent molecular ion. The sum of the dissociated fragments equals the parent and under given conditions will always fragment the same internal bonds to produce a predictable pattern (same ions and relative abundances for each). See also product (daughter) ions resulting from specific MRM experiments.
Gas phase ion: Analytes must be converted from their resting state to an ion in order to be manipulated and acquired by a mass spectrometer. There are a number of ways to accomplish this as described in the primer — some more aggressively creating fragments while others conserve the analyte intact. Energy is presented to the analyte, producing an ion in the gas phase as opposed to, for instance, the condensed phase used to separate analytes in LC.
Hybrid: Usually refers to an instrument that is a combination of two different types, for instance, earlier micromass "hybrids" combined magnetic sector and quadrupoles. Today's quadrupole time-of-flight (QTOF) instrument is a hybrid of quadrupole and TOF.
Ion: Mass spectrometers can only manipulate and therefore detect a mass when it possesses at least one charge. When only one charge is present (for instance, by the loss of an electron, causing the molecule to exist as a positively charged cation radical or by the addition of a proton or hydrogen to exist as a positively charged pseudomolecular ion), we can think of it as representing the molecular weight in a low-resolution scheme.
Ion current (total ion current): The electric current detected based upon the charged particles created in the ion source. If the mass spectrometer is set to scan over a range of 100–500 Da, the resulting total ion current will be the sum of all ions present in the source within that range at the selected time. If the instrument is set to detect only one ion (selected ion monitoring), the resulting total ion current will be the sum of only that ion at each selected instance.
Ion cyclotron resonance (ICR): Cyclotron instruments trap ions electrostatically in a cell using a constant magnetic field. Pulses of RF voltage create orbital ionic motion, and the orbiting ions generate a small signal at the detection plates of the cell (the ion's orbital frequency). The frequency is related inversely to the ions' mass-to-charge ratio (m/z), and the signal intensity is proportional to the number of ions of the same m/z in the cell. At very low cell pressures, a cyclotron instrument can maintain an ion's orbit for extended periods, providing very high resolution measurements. Fast-Fourier transform-ion cyclotron resonance (FT-ICR) instruments represent the extreme capability of measuring mass with the ability to resolve closely related masses. Although impractical for most applications, a 14.5-T magnet can achieve a resolution of more than 3.5 million and, thus, display the difference between molecular entities whose masses vary by less than the mass of a single electron.
Ion evaporation mechanism: Reference to ESI; in 1976, Iribarne and Thomson proposed the ion evaporation mechanism (see also "charge residue mechanism"), in which small droplets form by Coulombic fission, similar to the way they form in Dole's charge residue model. However, according to ion evaporation theory, the electric field strength at the surface of the droplet is high enough to make leaving the droplet surface and transferring directly into the gas phase energetically favorable for solvated ions.
Ion mobility: This technique differentiates ions based upon a combination of factors: their size, shape, and charge, as well as their mass. Ion mobility measurements and separations coupled with tandem mass spectrometry (MS) can help overcome analytical challenges that could not be addressed by other analytical means, including conventional MS or LC instrumentation. Ion mobility mass spectrometry (or IMMS because "imaging mass spectrometry" is often abbreviated IMS) devices are used commonly in airports and handheld field units for rapidly (20 ms) detecting small molecules whose mobility is known. A wide range of analytes are amenable to IMMS.
Ion source: The physical space in the ion stream in front of the analyzer, where the analyte is ionized. Each type of interface requires its own internal geometry for optimum results.
Ion trap: Also linear trap and Q-trap (or quadrupole trap). Wolfgang Paul's invention of the quadrupole and quadrupole ion trap earned him the Nobel Prize in physics in 1953. An ion trap instrument operates on principles similar to those of a quadrupole instrument. Unlike the quadrupole instrument, however, which filters streaming ions, the trap stores ions in a three-dimensional space. Before saturation occurs from too many ions attempting to fill the finite space, the trap or cyclotron allows selected ions to be ejected for detection. Fields generated by RF voltages applied to a stacked or "sandwich" geometry (end-cap electrodes at opposing ends) trap ions in space between the two electrodes. Ramping or scanning the RF voltage ejects ions from their secular frequency, or trapped condition. Dynamic range is sometimes limited. The finite volume and capacity for ions limits the instrument's range, especially for samples in complex matrices.
The ability to perform sequential fragmentation and, thus, derive more structural information from a single analyte (that is, fragmenting an ion, selecting a particular fragment, and repeating the process) is called MSn . GC peaks are not wide enough to allow more than a single fragmentation (MS-MS or MS2 ). Ion-trap instruments perform MS-MS or fragmentation experiments in time rather than in space, like quadrupole and sector instruments. So they cannot be used in certain MS-MS experiments such as neutral loss and precursor ion comparisons. Also, in MS-MS operation with an ion-trap instrument, the bottom third of the MS-MS spectrum is lost, a consequence of trap design. To counter the loss, some manufacturers make available via their software wider scan requirements that necessitate the switching of operating parameters during data acquisition.
Because of similarities in functional design, quadrupole instruments are hybridized to incorporate the advantages of streaming quadrupole and ion trapping behavior to improve sensitivity and allow on-the-fly experiments not possible with either alone. Such instruments are sometimes called linear traps or Q-traps). The increased volume of a linear trap instrument (over a three-dimensional ion trap) improves dynamic range.
Isotope ratio: Although often presumed to be constant and stable, natural isotope abundance ratios show significant and characteristic variations when measured very precisely. Isotope ratio measurements are useful in a wide range of applications, for example, metabolic studies using isotopically enriched elements as tracers; climate studies using measurements of temperature-dependent oxygen and carbon isotope ratios in foraminifers; rock age dating using radiogenic isotopes of elements such as lead, neodymium or strontium; and source determinations using carbon isotope ratios (for instance to determine if a substance occurs naturally or is a petroleum-based synthetic).
Typically, single focusing magnetic sector mass spectrometers with fixed multiple detectors (one per isotope) are used. Complex compounds are reduced to simple molecules before measurement — for example, organic compounds are combusted to CO2, H2O and N2.
Laser ablation: Compounds can be dissolved in a material that acts as an intermediate to transfer charge to the analytes of interest. A laser is aimed at the mixture to cause sputtering to produce ions in the space just above the mixture, where they can be sampled or drawn into the mass spectrometer. Especially useful as a very soft ionization technique to look at intact large molecules because the low-mass ions from the matrix produce a highly complex, intense background that could otherwise interfere with analytes of similar low mass.
Magnetic sector: Ions leaving the ion source are accelerated to a high velocity; they then pass through a magnetic field perpendicular to their direction. When acceleration is applied perpendicular to the direction of motion, the object's velocity remains constant, but the object travels in a circular path. Therefore, the magnetic sector is designed as an arc. Ions with a constant kinetic energy but different mass-to-charge ratio are brought into focus at the detector slit (called the "collector slit") at different magnetic field strengths.
The magnetic sector alone will separate ions according to their mass-to-charge ratio. However, because the ions leaving the ion source do not have exactly the same energy (and therefore do not have exactly the same velocity), the resolution will be limited. An electric sector that focuses ions according to their kinetic energy usually was added, which, like the magnetic sector, applies a perpendicular force to the direction of ion motion.
Matrix assisted laser desorption ionization (MALDI): First introduced in 1988 by Tanaka, Karas, and Hillenkamp, MALDI uses a laser to strike and energize a matrix containing the analyte. It has proven to be the method of choice for ionizing exceptionally large peptide and protein molecules that can then be detected intact. Commonly employed as the introduction scheme for TOF instruments, which are often referred to as MALDI-TOF.
Mass-to-charge ratio (m/z): Charged particles are represented as a ratio of their mass to their ionic charge. In literature and general use, this often appears as m/z, where the analyte from which the ion is derived might be labeled using atomic mass units (amu), daltons or molecular weight (mw).
Mean free path: The distance from entrance of an ion into the analyzer and detection of that ion. At operating vacuum, the mean free path is relatively long considering time between collisions in rarified air versus the time needed to analyze an ion. For example:
• atm (1000 torr) air contains
3 × 1022 molecules/cm3
• chamber at 1 × 10–5 torr
contains 3 × 1011 molecules/cm3
• λ √ pressure (torr) = minimum
mean free path (cm)
where λ = 5 × 10–3 cm.
Molecular ion: The ion produced when a molecule gains (anion) or loses (cation) an electron. See also pseudomolecular ion.
Monoisotopic mass: The monoisotopic mass of an element is the exact mass of the most abundant, naturally occurring, stable isotope. In a mass spectrometer able to report only to the nearest integer value, the molecular ion of a C50H102 compound might be represented by a peak at m/z 703 instead of at m/z 702, because the molecular ion would have a monoisotopic mass of 702.7825, which rounds to the integer 703. Once an ion exceeds a nominal mass of 1000 Da, there is no observed nominal m/z value peak in the mass spectrum. The monoisotopic mass peak is offset from where the nominal mass peak should be observed by an amount equal to the mass defect of the ion. For single-charge ions with masses above 500 Da, using techniques like electrospray with transmission quadrupole or quadrupole ion-trap mass spectrometers that have unit resolution throughout the m/z scale, the isotope peaks will be separated clearly. See also nominal mass.
Multiple reaction monitoring (MRM): A specific experiment on a triple-quadrupole mass spectrometer, in which a parent ion is filtered in the first quadrupole (Q1), a collision is then induced between the parent ion and a molecule (usually a gas such as argon) in the middle or "RF only" quadrupole (Q2), followed by detection of a specific product ion from that collision (Q3). Used in high-throughput quantitative analyses in the pharmaceutical industry especially.
MSn: A term coined with the resurgence of ion traps denoting the ability to choose a specific ion present in the ion source and fragment it; repeating the procedure to increase specificity when attempting to identify an analyte. The procedure can be repeated (number of repeats = n) providing the chosen fragments have sufficient energy and enough sample and time are provided for the experiment to continue.
Nominal mass: Electron ionization MS often relies upon perfluorinated compounds such as perfluorotributylamine (nominal molecular mass of 671) to calibrate the m/z scale. That is because the integer mass of an ion is almost the same as its monoisotopic mass. If an ion exceeds a nominal mass of 1000 Da, there is no observed nominal m/z value peak in the mass spectrum. The monoisotopic mass peak is offset from where the nominal mass peak should be observed by an amount equal to the mass defect of the ion. For single-charge ions with masses above 500 Da, using techniques like electrospray with transmission quadrupole or quadrupole ion trap mass spectrometers that have unit resolution throughout the m/z scale, the isotope peaks will be separated clearly. See also monoisotopic mass.
Open access (OA): Also referred to as "Walk-up systems," these are workflow controls allowing a fully trained operator to create complete LC– or GC–MS methods and make them available to a large number of nonspecialist users, giving them access to advanced technology without the requirement for extensive training.
Parent ion: More properly referred to as "precursor;" a generally interchangeable term with "molecular ion." Use of this term infers the presence of a product ion in an MRM scheme. See Product ion.
Particle beam: Originally developed at Georgia Tech and dubbed MAGIC (monodisperse aerosol generating interface for chromatography) by Browner and colleagues. The technique was later refined and is referred to generically as particle beam. The LC stream is heated and nebulized to remove the solvent. Vacuum pumps draw the solvent vapor through skimmer cones in series (usually two). The result is a "dried" particle that accelerates through the momentum separator and impacts the mass spectrometer source producing fragment ion spectra similar to traditional GC–MS. This technique is now rarely used.
Probe: Also solids probe or direct insertion probe. A metal rod inserted into the mass spectrometer source through a vacuum lock. Samples can be applied to the tip of the probe and placed into the path of an ionizing beam. Typically used for EI and other single sample manual experiments. Samples also can be applied in an ionization enhancing matrix as in the case of FAB.
Product ion: Formerly called "daughter ions," these are the result of controlled experiments in which a precursor (or "parent") ion and molecule collisions are induced to cause fragmentation. The collision gives rise to a product ion specific to the precursor ion and is used as a means of positive identification. See MRM.
Protonated molecular ion: Some forms of ionization produce ions by a proton transfer process that preserves and promotes the appearance of the molecular ion itself (the end result referred to as a pseudomolecular ion). In chemical ionization, for instance, the sample is exposed to an excess of reagent gas to form a protonated molecular ion (represented M+H). The reverse process can produce negative ions. Transferring the proton to the gas molecule can, in some cases, produce the negative ion (M-H) or deprotonated ion.
Pseudomolecular ion: Usually refers to the adduction of a proton (for example, M+H) or ion (for example, M+NH4 derived from the ammonium salt commonly used in the mobile phase) that alters the analyte of interest in some (relatively) easily identifiable fashion. The charge allows manipulation by the mass spectrometer.
Quadrupole: The underlying feature for the most prevalent type of mass spectrometer. Four rods (often no more than 1 in. in diameter and less than 12 in. long) are held parallel to each other (about 1 in. apart) in two collars. Filtering, or passing a given charged particle along its length, is accomplished by applying DC and RF voltage to the rods. Different masses (with associated charge) are affected by changing the RF–DC conditions. The rods are connected as paired opposites — each set alternated as the positive and negative poles by the RF source.
For a given calibrated setting, only particles of corresponding m/z will pass (approximately equivalent to molecular weight). The same setting will cause higher weight particles to miss the detector by passing in oblique fashion to the poles (the voltage settings having little or no effect) and lighter particles to become entrapped without reaching the exit and being detected. Quadrupoles can change and stabilize these mass filter field conditions quickly allowing more than one molecular weight to be observed by scanning over time, although fewer charged particles are therefore detected for any given molecular weight.
Functionally, the single-quadrupole mass filter, used alone when matrix interference is not an issue, can be joined with another to enhance discrimination of a given analyte among many in a background (from the matrix for instance). Early designs used a third quadrupole-type device between the two as the collision cell (hence, the term "triple quadrupole"), while more recent designs use specialized devices and are referred to as "tandem quadrupoles."
Quadrupole time-of-flight (QTOF): This mass spectrometer couples a TOF instrument with a quadrupole instrument. This pairing results in the best combination of several performance characteristics: accurate mass measurement with a TOF instrument and the ability to carry out fragmentation experiments between the two along with high-quality quantitation and mass filtering. A QTOF instrument's high mass accuracy falls within a few parts per million of the true, calculated, monoisotopic value, and its high resolution — as much as 10 times higher than a quadrupole instrument's — permits determination of empirical formulas according to mass defect (where the critical mass value of hydrogen and other atoms present serve as a differentiator).
Radical cation: Most stable organic compounds have an even number of total electrons because electrons occupy atomic orbits in pairs. When a single electron is removed from a molecule, the total electron count becomes an odd number, a radical cation. The molecular ion in a mass spectrum is always a radical cation (as seen in EI), but the fragment ions can be even-electron cations or odd-electron radical cations, depending upon the neutral (uncharged) fragment lost. The simplest and most common fragmentations are bond cleavages that produce a neutral radical (odd number of electrons), and a cation having an even number of electrons. A less common fragmentation where an even-electron neutral fragment is lost produces an odd-electron radical cation fragment.
Radio frequency: see "Direct current."
Resolution (10% valley method): The minimum separation between two neighboring masses of approximately equal response for the mass spectrometer to distinguish between ions of different mass-to-charge ratio. More typically used with magnetic sectors; equal to the average mass of the two particles divided by the difference in their masses.
Resolution (M/ΔM): More commonly used as a measure in which a given mass is divided by the resolution at full width half height maximum (FWHM). The 10% valley method was prevalent with magnetic sector instruments and requires that the neighboring masses be of equal intensity. For instance, a typical resolution value for a quadrupole is 0.6 amu at FWHM. Measured using an acquired peak at m/z 3000 (equivalent to daltons or amu) equals a resolution of 5000. The results of the two techniques are roughly comparable, with this method typically yielding values double the valley method. Equal to the mass divided by the peak width at 50% peak height.
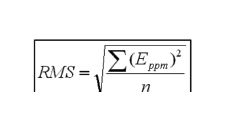
Root-mean square (RMS) error measurement: A comprehensive method of evaluating instrument mass accuracy measurement capability, which resembles intended use by calculating the RMS error. The RMS error is calculated using the following relation, in which Eppm is the parts-per-million error and n is the number of masses considered:
Scanning: See also selected ion monitoring, quadrupole and ion current. Control voltages (DC and RF) are adjusted by the computer over a given time to scan (detect) any charged particles in the specified range. The benefit of being able to detect more than one species is at the expense of sensitivity because some of the desired particles undoubtedly will be available to the detector while it is set to detect elsewhere in the range.
Selected ion monitoring (SIM): Also called selected ion recording (SIR); refers also to quadrupole and scanning. The DC and RF voltage settings on the quadrupoles can be adjusted to pass only one charged particle (a single mass-to-charge ratio) through to the detector. The result is a dramatic decrease in noise, allowing the signal to appear as a dramatic increase in sensitivity (all particles of that m/z are being detected all the time) at the expense of any other particles in the mixture being detected at all.
Thermospray: Although this type of interface has been in the literature for some time, it was popularized in the early 1980s. Vestal and Blakely should be given credit for creating the first true commercially feasible interface between LC and MS. LC solvent at a flow rate of approximately 1 mL/min is heated in a probe (insulated tubing approximately 1–2 ft long and 75–150 μm internal diameter) and the resulting vapor is sprayed into the mass spectrometer. Ions created by the desolvation of the aerosol droplets inside the mass spectrometer enter the analyzer (at right angles to the spray) and are affected by the lens voltages (see scanning, total ion current).
The spectra produced are termed soft-ionization spectra because little meaningful fragmentation is produced. An intense molecular ion is produced and while the single ion can be of little advantage in some backgrounds and mixtures, it is advantageous for high molecular weight confirmation at great sensitivity and for filtering a target ion for further fragmentation (MS-MS). Literature reports low picomole sensitivity for vitamin D metabolites. The interface was chosen generally for highly polar applications such as metabolite work before the refinement of APCI in the early 1990s, and it worked poorly as organic content in the liquid increased.
Time-of-flight (TOF) mass spectrometer: A mass analyzer that separates ions of different mass-to-charge ratios by their time of travel through a field-free vacuum region after having been given the same kinetic energy. The velocity of the ions is dependent upon their mass-to-charge ratio and as the ions are traveling over a fixed distance, the time taken to reach the detector allows the mass-to-charge ratios to be determined, with heavier ions taking longer.
Tuning: Typically refers to optimizing the interface lenses and flowing gases to achieve a desired response for a specific analyte under a set of operating conditions as opposed to calibration. Calibration defines the mass acquisition and reporting function. Hardware settings of lenses and related circuits in conjunction with creation of a software look-up table sets a stable instrument's response, corrected to a list of known masses from a flowing stream of calibrant such as polyethylene glycol (PEG) or NaCsI.
Vacuum (torr): Equivalent to 1 mm Hg (1 psi = 51.7 torr = 0.069 bar or atm). The analyzer portion of the mass spectrometer typically must be maintained at a minimum of 10–4 torr to allow discrete passage of the ionized particles. Pressures tending toward atmospheric cause ion–molecular interactions that can produce random results in the charged particles being detected further downstream. Under controlled conditions, such collisions are induced at low vacuum (higher pressure) for techniques such as CI.
Michael P. Balogh
"MS — The Practical Art" Editor Michael P. Balogh is principalscientist, MS technology development, at Waters Corp. (Milford, Massachusetts); a former adjunct professorand visiting scientist at Roger Williams University (Bristol, Rhode Island); cofounder and current president of the Society for Small Molecule Science (CoSMoS) and a member of LCGC's editorial advisory board.

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).
Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









