E-Cigarette Vapours for Regulatory Compliance and Quality Control
The Application Notebook
This study describes a simple, quick approach for the sampling and analysis of nicotine, impurities, and flavour compounds in e-cigarette vapours. Combining thermal desorption (TD) with gas chromatography–mass spectrometry (GC–MS) analysis results in a versatile screening method for tackling the challenge of regulatory compliance and quality control in this rapidly expanding industry.
Hannah Calder, Caroline Widdowson, and David Barden, Markes International
This study describes a simple, quick approach for the sampling and analysis of nicotine, impurities, and flavour compounds in e-cigarette vapours. Combining thermal desorption (TD) with gas chromatography–mass spectrometry (GC–MS) analysis results in a versatile screening method for tackling the challenge of regulatory compliance and quality control in this rapidly expanding industry.
The Need to Analyze E-Cigarette Vapours
E-cigarettes have seen a massive growth in popularity in recent years, and this has led agencies to tighten up regulatory requirements - specifically the EU Tobacco Products Directive (TPD), and an extension of the authority of the US Food and Drug Administration over regulation of tobacco products to include e-cigarettes.
These developments require e-cigarette manufacturers to submit information both on the chemical constituents of “e-liquids” and of the emissions resulting when e-cigarettes are activated. The compounds stipulated include major components such as nicotine and diethylene glycol, as well as minor components giving rise to health concerns, such as those resulting from combustion.
As well as regulatory compliance, VOC-profiling of e-liquids or e-cigarette vapour is valuable for quality control and product improvement - for example, by checking the consistency of levels of nicotine and flavour chemicals, or checking for contaminants.
In this investigation, we show how a simple, inexpensive approach employing sorbent tubes to sample organic vapours from e-cigarettes can be interfaced with TD–GC–MS for the rapid analysis of target VOCs and SVOCs. The result is a protocol that incorporates simple sampling, robust and automated analysis, flexibility in setting method parameters, and comprehensive data analysis, making it a simple solution to the challenge of regulatory compliance and quality control.Experimental Procedure
An e-cigarette was connected to a 3½” × ¼” o.d. “Odour/Sulphur” inert-coated stainless steel sorbent tube. This assembly was then connected to an Easy-VOC™ grab-sampler, the e-cigarette activated, and 50 mL of vapour collected over a period of 4 s (Figure 1).
Figure 1: Schematic showing assembly used to sample e-cigarette vapours.
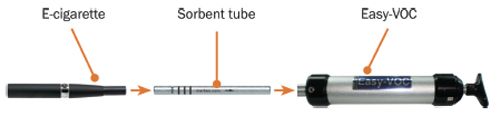
Analysis of the tubes was carried out by TD–GC–MS, using a TD100âxr™ thermal desorber, a ZBâ35HT column (tobacco-flavoured eâliquid) or a VF-624ms column (menthol and blueberry-flavoured eâliquid), and a quadrupole mass spectrometer used in scan mode.
For full analytical conditions, please see Markes International Application Note 118.
Analysis of E-Cigarette Vapours
The vapours released from the e-cigarette were analyzed in two separate studies with different-flavoured e-liquids. In both cases an Easy-VOC grab-sampler was used to pull the vapours from the e-cigarette directly into a sorbent tube, followed by TD–GC–MS analysis.
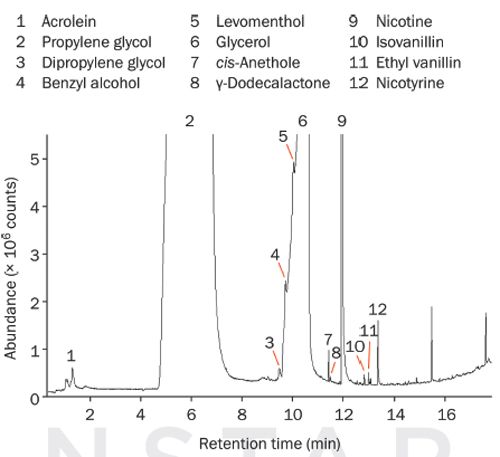
The first study used a “tobacco-flavoured” e-liquid (Figure 2), and found a number of trace-level components despite the very high concentrations of propylene glycol (#2) and glycerol (#6). These components included acrolein (#1), which (as well as nicotine [#9]) is present on both the EU and FDA lists of compounds of concern, and flavour/aroma compounds that may be of relevance to the consumer experience, such as levomenthol (#5) and ethyl vanillin (#11).
Figure 2: TD-GC-MS analysis of vapour from an e-cigarette loaded with a tobacco-flavoured e-liquid, indicating major components and some trace-level components.
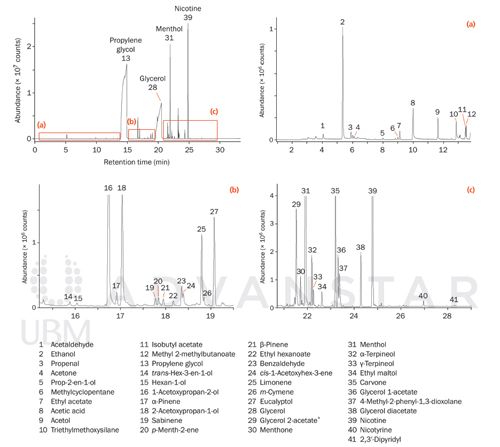
The second study used an e-liquid with a “menthol and blueberry” flavour. Initially, a high outlet split (that is, with a small proportion of the sample sent to the GC) was used to quantify the higher-loading compounds, including menthol (#31) and nicotine (#39), followed by a lower outlet split of the re-collected sample (Figure 3). This ensured a strong response from the lower-level flavour compounds, increasing their intensity relative to the heavily overloaded peaks for propylene glycol and glycerol.
It is worth noting that the use of inert-coated stainless steel tubes, combined with the inertness of the flow-path in Markes’ TD instruments, minimizes the loss of thermally labile species, such as sulphur compounds and monoterpenes (for example, α-pinene [#17], sabinene [#19], and β-pinene [#21] indicated in Figure 3).
Figure 3: TD-GC-MS analysis of vapour from an e-cigarette loaded with a menthol and blueberry flavour e-liquid, indicating major components and a selection of lower-level components (some with regulatory or organoleptic relevance). * = Identity tentative.
Conclusions
This study has shown that sampling of e-cigarette vapours onto sorbent tubes using the Easy-VOC, followed by TD–GC–MS analysis, is a simple and easy approach that is suitable for assisting compliance with e-cigarette regulations by allowing the detection of a wide range of target VOCs and SVOCs. The same approach, because of the speed of sampling and analysis, is also valuable for quality-control and R&D in this and other food/fragrance applications.
A valuable feature of Markes’ TD instruments (including the TD100-xr used here) is the ability to automate re-collection of samples on the inlet and/or outlet splits. This can be used, for example, to analyze a single sample using multiple methods, which has obvious advantages for method development.

Markes International
Gwaun Elai Medi-Science Campus, Llantrisant, Wales, UK
Tel.: 44 (0)1443 230935
E-mail: enquiries@markes.com
Website: www.markes.com

Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











