HILIC–UHPLC–MS as a Tool for Metabolomics Study
The Application Notebook
The rising numbers of liquid chromatography–mass spectrometry (LC–MS)-based metabolomics applications and untargeted metabolism studies have increased the need for reliable chromatographic separations for a large quantity of molecules with widespread polarities.
Matthias Cuykx1, Wen Jiang2, and Adrian Covaci1, 1Toxicological Centre, University of Antwerp, Antwerp, Belgium, 2HILICON AB
The rising numbers of liquid chromatography–mass spectrometry (LC–MS)-based metabolomics applications and untargeted metabolism studies have increased the need for reliable chromatographic separations for a large quantity of molecules with widespread polarities.
Hydrophilic interaction liquid chromatography (HILIC) is an emerging MS-compatible separation technique that retains polar compounds through multiple retention mechanisms (1). Its inverse retention characteristics compared to reversed-phase liquid chromatography (LC) offers an advantage in separation of polar metabolites (2). However, it is not an easy task to select the right HILIC stationary and mobile phase system as a tool to accomplish successful studies in metabolomics.
In this application, we used a 1.8-µm iHILIC-Fusion UHPLC HILIC column packed with charge modulated hydroxyethyl amide silica. Its separation principle combines hydrophilic partitioning, weak electrostatic interactions, and hydrogen bonding.
Experimental
LC–MS/MS system: Agilent 1290 Infinity UHPLC system with an Agilent 6530 QTOF mass spectrometer
Column: 100 × 2.1 mm, 1.8-µm, 100 Å, iHILIC-Fusion (P/N 110.102.0110, HILICON AB) at 30 °C
Gradient elution: A) acetonitrile/methanol (98/2, v/v); B) 10 mM ammonium formate with 0.1% (v/v) formic acid (pH 3.15) in ultraâpure (Milli-Q) Water
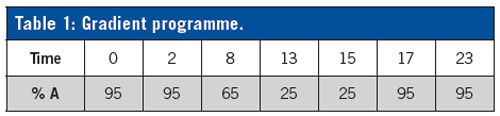
Flow rate: 0.3 mL/min
Injection volume: 2 µL
MS system setting:
Drying gas: 250 °C at 8 L/min
Sheath gas: 350 °C at 11 L/min; Nebulizer: 45 psig
Voltages: Capillary: 2000 V; Fragmentor: 150 V
Sample preparation: Standard solutions were prepared as 1 µg/mL in 60/40 (v/v) acetonitrile/Milli-Q water for estimation of retention times. The physicochemical range of our selected metabolites was broad enough to guarantee a reliable (non-lipid) metabolomics separation.
HepaRG cells (Biopredic) were used for cell culture on 12-well plates. The cells were scraped with an 80% methanol aqueous solution from each well and applied for liquid–liquid extraction using 1/1.5/1 methanol/water/chloroform mixture. The polar phase was collected and evaporated to dryness and then reconstituted with 60/40 (v/v) acetonitrile/Milli-Q water.
Results and Discussion
The normalized chromatograms of a mixture of 11 standards are shown in Figure 1. We observed as a general elution trend that the retention time increases with increasing polarity of compound. Interestingly, we found a remarkable separation between leucine and isoleucine (5 and 6), which are normally difficult to be separated with other HILIC columns because they only differ in the position of a methyl group in the alkane chain.
Figure 1: HILIC-MS chromatographic separation of standards: 1. Misoprostol 2. Caffeine 3. Adenine 4. Phenylalanine 5. Leucine 6. Isoleucine 7. Serine 8. Tyrosine 9. Folic acid 10. Lysine 11. Ornithine.
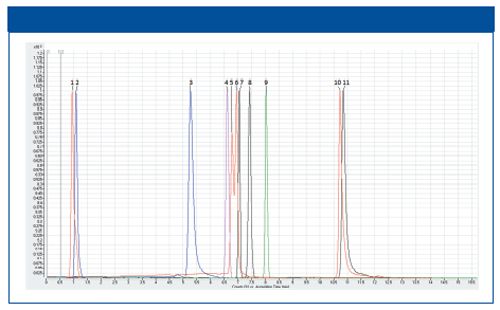
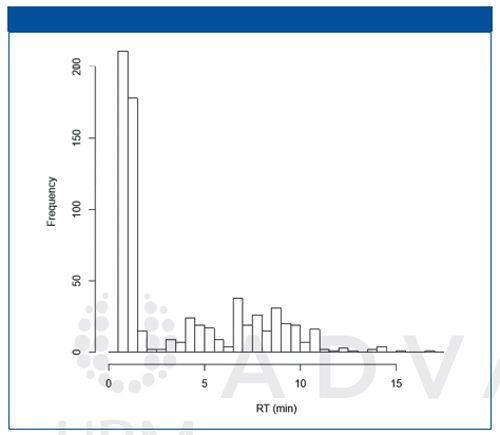
The separation of cell extracts was confirmed by chromatograms of the injected standards. It was found that relatively non-polar compounds were eluted at first; neutral polar compounds eluted between 3 and 10 min; and polar basic compounds eluted at 10–15 min. In summary, our newly developed method can detect 700 molecules within a 15 min gradient run.
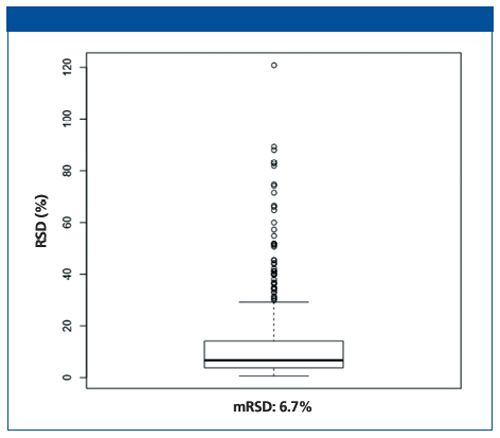
The assessment of the quality of the chromatograms was not straightforward in LC–MS metabolomics since the identity of most compounds was not known a priori. Manual evaluation of these compounds was thus impossible. We used a quality assurance strategy with repeated injections of a QC-sample (3). The precision of measuring the extracted metabolites across these QC-injections was evaluated by calculating the relative standard deviations (RSD). The RSDs obtained for each compound were evaluated as a population representing the entire dataset, and the calculated median RSD (mRSD) expresses the quality of the LC–MS performance in a single value (4).
Figure 2: Numbers of detected features from the chromatograms of a polar HepaRG cell extract.
As shown in Figure 3, with iHILIC-Fusion, the mRSD was 6.7% and 70% of the variables had a RSD below 15%. According to the regulation by FDA, an RSD below 20% is considered acceptable (5). Hence, the current
HILIC–UHPLC–MS setup provided an excellent detection system that can be used for the analysis of polar compounds for metabolomics purposes.
Figure 3: Metabolomics variance of the extracted features.
Conclusion
The iHILIC-Fusion column has a mixed separation principle that can efficiently separate a large quantity of polar metabolites based on their multiple physicochemical properties. Accordingly, its separation efficiency of intra-class metabolites is higher than other HILIC columns used in our tests.
References
- P. Jandera, Anal. Chim. Acta.692, 1–25 (2011). doi:10.1016/j.aca.2011.02.047.
- S.U. Bajad, W. Lu, E.H. Kimball, J. Yuan, C. Peterson, and J.D. Rabinowitz, J. Chromatogr. A. 1125, 76–88 (2006). doi:10.1016/j.chroma.2006.05.019.
- W.B. Dunn, I.D. Wilson, A.W. Nicholls, and D. Broadhurst, Bioanalysis4, 2249–64 (2012). doi:10.4155/bio.12.204.
- H.M. Parsons, D.R. Ekman, T.W. Collette, and M.R. Viant, Analyst134, 478–485 (2009). doi:10.1039/b808986h.
- T. Cajka and O. Fiehn, Anal. Chem. 88 (2016). acs.analchem.5b04491. doi:10.1021/acs.analchem.5b04491.

HILICON AB
Tvistevägen 48, SE-90736 Umeå, Sweden
Tel.: +46 (90) 193469
E-mail: info@hilicon.com
Website: www.hilicon.com

Assessing Thorium-Peptide Interactions Using Hydrophilic Interaction Liquid Chromatography
February 4th 2025Paris-Saclay University scientists used hydrophilic interaction liquid chromatography (HILIC) coupled to electrospray ionization mass spectrometry (ESI-MS) and inductively coupled plasma mass spectrometry (ICP-MS) to assess thorium’s interaction with peptides.









