Implementation of Highly Efficient Protein Characterization and Preparative iCIEF
The Application Notebook
Whole column imaging detection capillary isoelectric focusing (iCIEF) has been recognized as a powerful tool for biopharmaceutical development and quality control. Unlike conventional single point detection capillary electrophoresis (CE) systems, in which light absorbance or emission at a specific point along the separation capillary is monitored, iCIEF detects a line of light that is passed through and radiates from the entire separation capillary. As a result, sequential snapshots of the whole separation capillary at different times are obtained. This allows sample separation and interaction in real time to be observed during electrophoresis, enabling fast analytical method development, high resolution separation, and high sample throughput.
Advanced Electrophoresis Solutions Ltd.
Whole column imaging detection capillary isoelectric focusing (iCIEF) has been recognized as a powerful tool for biopharmaceutical development and quality control. Unlike conventional single point detection capillary electrophoresis (CE) systems, in which light absorbance or emission at a specific point along the separation capillary is monitored, iCIEF detects a line of light that is passed through and radiates from the entire separation capillary. As a result, sequential snapshots of the whole separation capillary at different times are obtained. This allows sample separation and interaction in real time to be observed during electrophoresis, enabling fast analytical method development, high resolution separation, and high sample throughput.
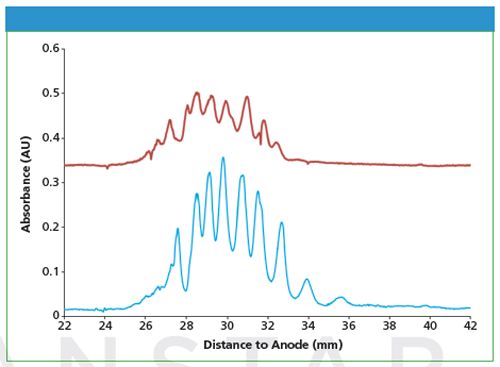
The detection sensitivity of iCIEF is determined by the detection wavelength and the length of the detection light path. The detection wavelength for normal capillary isoelectric focusing (CIEF) is 280 nm because of the relatively strong background of carrier ampholytes (a synthetic amphoteric mixture that forms a pH gradient under an electric field). Using a large inner diameter (i.d.) separation capillary allows for improved detection sensitivity before the commercial availability of low UV 214 nm carrier ampholytes. Recently, a CEInfinite cartridge series with 200 µm i.d. capillaries has been developed. These cartridges are shown to have various advantages such as low resistance for sample injections and less cartridge clogging, in addition to a sensitivity improvement over cartridges with a 100-µm i.d. capillary (refer to Figure 1).
Figure 1: Separation of a fusion protein sample with different internal diameter cartridges (red trace, 100 micrometer capillary; blue trace, 200 micrometer capillary). iCIEF method conditions: 1 min focusing period using 1500 V, 5 min focusing period using 3000 V; detection at 280 nm. Sample conditions: 0.2 mg/mL fusion protein in 1% Aeslyte HR 3 -10, 3% HR 6 - 7.5, 4M urea.
The resolution of an iCIEF system is mainly determined by the quality of the optical imaging subsystem, including the imaging sensor and focusing lens. It may also be affected by the scan rate of the imaging sensor. Imaging blur can occur as the result of slow scanning of a moving object. The application of a highly sensitive and high scan rate scientific CMOS imaging sensor can effectively improve the resolution of an iCIEF system, enabling investigation of dynamic processes with CEInfinite.
The isoelectric point (pI) of a protein is useful for protein identification and quality control. The pI of a protein can be calibrated conveniently with two pI markers in iCIEF. When used under enzymatic conditions, non-peptide synthetic small molecule pI markers are preferred to avoid losing peaks as would occur with peptide pI markers. The CEInfinite product line includes the most comprehensive, well spaced, and well calibrated pI markers on the market, covering a broad pH range from 2.5 to 10.5.
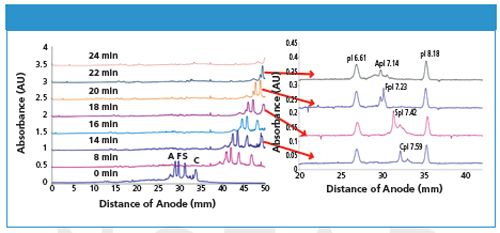
The successful application of iCIEF for charge-based protein profiling, identification, and potency assays has raised the need to further characterize protein charge variants with orthogonal techniques. Preparative iCIEF and iCIEF coupled to mass spectrometry (iCIEF-MS) are two highly demanded techniques, which can be accomplished using the CEInfinite system along with its proprietary separation cartridges. One example is fractionation of haemoglobin variants with CEInfinite (refer to Figure 2). The focused protein zones inside the separation capillary were continuously pushed out to the outlet transfer capillary after the desired CIEF separation, and collected into different vials. The high resolution CIEF separation achieved with the larger inner diameter separation capillary is maintained, as each protein zone is transferred into the smaller inner diameter transfer capillary. The collected protein variants (haemoglobin AFSC) were analyzed with iCIEF to confirm the resolution of the fractionation. The purified proteins can also be characterized with UHPLC–MS, and molecular weight and structure information can be obtained. Better fractionation resolution can be expected using shorter collection intervals. The separation capillary completes the electrical circuit for CIEF. As a result, the eluent out of the transfer capillary is separated from the electrical current - this makes it simpler and more straightforward to couple CEInfinite to a mass spectrometer when compared to conventional CE.
Figure 2: Preparative iCIEF and confirmation of fractionation collection with CEInfinite.

Advanced Electrophoresis Solutions Ltd.
1600 Industrial Rd, Cambridge, ON, N3H 4W5, Canada
Tel.: 1 518 653 6888 Fax: 1 519 804 4266
E-mail: sales@aeslifesciences.com
Website: www.aeslifesciences.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












