Seamless Method Transfer from an Agilent 1100 Series Quaternary LC to an Agilent 1260 Infinity II LC
The Application Notebook
This application note describes the transfer of a conventional LC method for the analysis of antihistaminic drugs from an Agilent 1100 Series Quaternary LC to an Agilent 1260 Infinity II LC, and demonstrates that equivalent results can be obtained.
Sonja Krieger, Agilent Technologies Inc.
This application note describes the transfer of a conventional LC method for the analysis of antihistaminic drugs from an Agilent 1100 Series Quaternary LC to an Agilent 1260 Infinity II LC, and demonstrates that equivalent results can be obtained.
Introduction
Instrument-to-instrument method transfer is an important topic for all laboratories throughout different industries (1). One example is the transfer of conventional LC methods from older equipment such as the Agilent 1100 Series Quaternary LC, to new instruments such as the Agilent 1260 Infinity II LC. This application note (2)describes the analysis of antihistaminic drugs using an 1100 Series Quaternary LC. The method is transferred without any changes to the 1260 Infinity II LC, and equivalent results in terms of retention time and resolution are obtained. Furthermore, the conventional LC analysis of antihistaminic drugs can be transferred to UHPLC conditions, optimized for resolution as well as for speed, using the Agilent 1260 Infinity II LC.
Experimental Conditions
Conventional LC analysis of the antihistaminic drugs tripelenamine, chlorpheniramine, tetracaine, and promethazine was achieved with the Agilent 1100 Series Quaternary LC as well as the Agilent 1260 Infinity II LC. An Agilent ZORBAX SB-C18 column (4.6 × 150 mm, 5-μm) was used with a gradient of 25 mM potassium dihydrogen phosphate in water (pH 3) and acetonitrile at a flow rate of 1.5 mL/min and a temperature of 40 °C. Diode array detection was performed at 204 nm.
Results
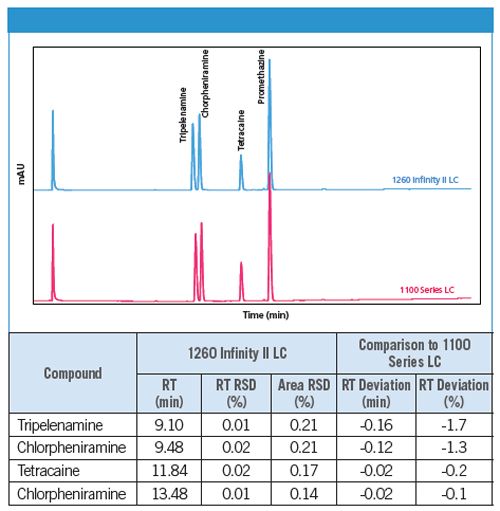
The conventional LC analysis of antihistaminic drugs was transferred without any method changes from an Agilent 1100 Series Quaternary LC to an Agilent 1260 Infinity II LC. By using the Agilent 1260 Infinity II LC, excellent retention time and area precision was achieved. Figure 1 compares the retention times of the antihistaminic drugs obtained using the Agilent 1100 Series Quaternary LC and the Agilent 1260 Infinity II LC. With a maximum deviation of -1.7%, excellent agreement of retention times was observed. Further, a slight increase in resolution was achieved using the Agilent 1260 Infinity II LC. This proves the equivalency of the 1260 Infinity II LC compared to the 1100 Series Quaternary LC for the analysis of antihistaminic drugs.
Figure 1: Conventional LC analysis of antihistaminic drugs on an Agilent 1100 Series Quaternary LC and an Agilent 1260 Infinity II LC.Figure 1: Conventional LC analysis of antihistaminic drugs on an Agilent 1100 Series Quaternary LC and an Agilent 1260 Infinity II LC.
With a pressure range of up to 600 bar, the Agilent 1260 Infinity II LC also enables UHPLC analyses to be performed using Agilent InfinityLab Poroshell columns. The transfer of the conventional LC analysis of antihistaminic drugs to UHPLC conditions, optimized for resolution as well as for speed, offers the option to increase peak resolution and at the same time reduce analysis time and solvent use.
Conclusions
The transfer of a conventional LC method for the analysis of antihistaminic drugs from an Agilent 1100 Series Quaternary LC to an Agilent 1260 Infinity II LC showed a maximum retention time deviation of -1.7% as well as a slight increase in resolution, and thereby proves the equivalency of the 1260 Infinity II LC compared to the 1100 Series Quaternary LC for the analysis of antihistaminic drugs.
References
- Agilent 1290 Infinity with ISET, Agilent Technologies User Manual, part number G4220-90314 (2015).
- S. Krieger, Method Transfer from an Agilent 1100 Series Quaternary LC to an Agilent 1260 Infinity II LC, Agilent Technologies Application Note, publication number 5991-6914EN (2016).

Agilent Technologies, Inc.
5301 Stevens Creek Blvd., Santa Clara, California 95051, USA
Tel: (800) 227 9770
Website: www.agilent.com

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).
Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











