Determination of Steviol Glycosides by HPLC with UV and ELS Detections Using the Acclaim Mixed-Mode WAX-1 Column
The stevia plant and its extracts have long been used as sweeteners in Asia and Latin America. Two steviol glycosides present in plant tissue, stevioside, and rebaudioside A, are largely responsible for the sweet flavor (1).
Deanna C. Hurum, Brian M. De Borba, and Jeffrey S. Rohrer, Dionex Corporation
The stevia plant and its extracts have long been used as sweeteners in Asia and Latin America. Two steviol glycosides present in plant tissue, stevioside, and rebaudioside A, are largely responsible for the sweet flavor (1). In December 2008, the US FDA placed rebaudioside A (also known as rebiana) on the Generally Recognized as Safe (GRAS) list of sugar substitutes to be used in foods, thereby allowing the use of rebiana as a commercial sweetener (2). The determination of steviol glycosides in these sweeteners is challenging due to their weak UV absorbance. Other detection methods, such as evaporative light scattering (ELS), can improve steviol glycoside quantification. In this proposed method, steviol glycosides were determined by UV and ELS detections in consumer sweeteners following separation on the Acclaim Mixed-Mode WAX-1 column (3).
Experimental
A Dionex UltiMate® 3000 system with an HPG-3200M pump, a TCC-3200 column compartment, a WPS-3000TSL micro autosampler, a VWD-3400 detector, and a Varian 380-LC ELS detector were used. An Acclaim® Mixed-Mode WAX-1, 5 μm (2.1 × 150 mm) column was used for all separations. Chromeleon® Chromatography Data System was used for system control and data processing.
Results and Discussion
Figure 1 shows the separation of a sweetener derived from stevia leaves and dispersed in inulin. Multiple glycosides are detected in this product. When analyzed by UV (Figure 1A), the detection method in the JECFA and USP monographs, four steviol glycosides are easily determined: dulcoside A, stevioside, rebaudioside C, and rebaudioside A. When the sample is analyzed using ELS detection (Figure 1B), there are a number of additional peaks not observed by UV. Rebaudioside B is present in the sample, which was not apparent by UV detection. Method precision was tested by replicate injections of standards using two different batches of mobile phase. Peak area RSDs range from 0.74 to 1.98 for stevioside and rebaudioside A. Retention time precision is excellent with RSDs ranging from 0.05 to 0.14. Retention time variability between batches of mobile phase was within 1.1% for rebaudioside A. This data indicates that the method is reproducible.
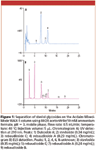
Figure 1
Conclusion
In this method, UV and ELS detections are used to determine steviol glycosides in a consumer sweetener. The Acclaim Mixed-Mode WAX-1 column is used in the HILIC mode to resolve the steviol glycosides. The method proposed requires only 12.5 mL of mobile phase sample compared to 25 to 60 mL/sample in the JECFA and USP methods, respectively. This saves time and resources during mobile phase preparation, and reduces waste. In addition, ELS detection distinguishes additional components in the sample that are not UV absorbing, thus allowing for an additional method to check sweetener purity.
References
(1) Prakash, I., DuBois, G.E.; Clos, J.F.; Wilkens, K.L.; Fosdick, L.E. Food and Chemical Toxicology 46, S75–S82 (2008).
2) US Food and Drug Administration, Agency Response Letter to GRAS Notice No. GRN 000253, Dec 17, 2008.
3) Dionex Corporation. Application Note 241, LPN 2371, Dionex Corporation, Sunnyvale, CA, (2009).
Acclaim, UltiMate, and Chromeleon are registered trademarks of Dionex Corporation.
Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA
tel. (408)737-0700; fax (408)730-9403
Website: www.dionex.com
















