Current Advances in Microextraction by Packed Sorbent (MEPS) for Bioanalysis Applications
Microextraction by packed sorbent (MEPS) is a new technique in sample preparation that can be connected on-line to gas chromatography (GC) or liquid chromatography (LC) without any modifications.
Microextraction by packed sorbent (MEPS) is a new technique in sample preparation that can be connected on-line to gas chromatography (GC) or liquid chromatography (LC) without any modifications. MEPS is a miniaturized, solid-phase extraction (SPE) technique that works with sample volumes as small as 10 µL The key aspect of MEPS is that the solvent volume used for the elution of the analytes is of a suitable order of magnitude to be injected directly into GC or LC systems. MEPS significantly reduces the volume of solvent and sample needed. This new technique offers significant advantages in terms of speed and simplicity and could be of interest in clinical, forensic toxicology and environmental analyses. The application of MEPS to clinical and preclinical studies will be described in this paper.
Introduction
The measurement of drug and metabolite levels in plasma and blood is of paramount importance in the drug discovery and development process. The more rapidly these measurements can occur, the more quickly drugs can progress towards regulatory approval. Off-line sample preparation is a limiting step for achieving fast bioanalysis, indicating a clear need for on-line sample preparation to speed-up the process. Biological fluids, such as plasma and urine, are much more complex than many others due to the presence of proteins, salts and various organic compounds with a similar chemistry to the analytes of interest. Thus, the extraction methods for biological samples have been difficult. Also, the matrix problems may call for specific skills in quantitative bioanalysis, particularly in electrosparay ionization mass spectrometry (ESI-MS). Signal suppression caused by unknown matrix interferences is often observed. Changes in the sample pretreatment procedures may solve the problem. A good sample preparation method will effectively isolate the analyte of interest from a complex matrix for quantification. It will enhance selectivity and sensitivity. It will eliminate the ion suppression in electrospray ionization mass spectrometry (ESI-MS). High accuracy and precision will also be obtained.
Microextraction by packed sorbent (MEPS) is a new development in the field of sample preparation and sample handling. It entails the miniaturization of conventional solid-phase extraction (SPE) packed-bed devices from millilitre bed volumes to microlitre volumes. MEPS can be connected on-line to GC or LC without any modifications. In MEPS approximately 1 mg of the solid packing material is packed inside a syringe (100–250 μL) as a plug or between the barrel and the needle as a cartridge. Sample preparation occurs on the packed bed. The bed can be coated to provide selective and suitable sampling conditions. MEPS differs from commercial SPE in that the packing is integrated directly into the syringe and not into a separate column. Thus, there is no need for a separate robot to apply the sample as in the case of conventional SPE. Moreover, the packed syringe can be used several times, more than 100 times using plasma or urine samples, whereas a conventional SPE column is used only once. MEPS can handle small sample volumes (10 μL plasma, urine, or water) as well as large volumes (1000 μL) and can be used for gas chromatography (GC) and liquid chromatography (LC) applications. The MEPS approach to sample preparation is suitable for normal phases, reversed phases, mixed mode and ion exchange chemistries. Because MEPS can be used for sample volumes as small as 10 μL, it can be used on-line with liquid chromatography mass spectrometry (LC–MS) analysis of volume-limited samples. MEPS can be fully automated — the sample processing, extraction and injection steps are performed online using the same syringe. The superior performance of MEPS was recently illustrated by on-line LC–MS and GC–MS assays of drugs and metabolites in water, urine, plasma and blood samples.1–24
The combination of MEPS and liquid chromatography mass spectrometry (LC–MS) is a good tool for the screening and determination of drugs and metabolites in blood, plasma and urine samples. MS is currently one of the most powerful detection techniques, particularly in pharmaceutical analysis, where good selectivity and high sensitivity are often needed. MEPS significantly reduces the volume of solvent and sample needed. This approach to sample preparation is very promising for many reasons: (1) it is easy to use, (2) it is a fully automated on-line procedure, (3) it is rapid and (4) the cost of analysis is minimal compared with conventional SPE.
The MEPS technique has been used to extract a wide range of analytes in different matrices (urine, plasma, blood). Hence, several drugs such as local anesthetics and their metabolites,1–5,9,10,12 the anticancer drugs roscovitine, olomoucine, busulphan, cyclophosphamide and AZD3409,6–8,18–20 the β-blockers acebutolol and metoprolol,15 the neurotransmitters dopamine and serotonin,16 methadone17 and cocaine and cocaine metabolites23 have been extracted from biological samples such as blood, plasma or urine samples using MEPS.
Microextraction by Packed Sorbent (MEPS)
Description of MEPS: MEPS is a new miniaturized, SPE technique that can be connected on-line to GC or LC without any modifications. It was invented and developed at AstraZeneca, Södertälje, Sweden.1,2 MEPS works with much smaller samples (as small as 10 μL) than full-scale SPE. It can be fully automated — the sample processing, extraction and injection steps are performed on-line using the same syringe. It significantly reduces the volume of solvent and sample needed. In MEPS the sorbent, 1–2 mg, is either inserted into the syringe (100–250 μL) barrel as a plug [Figure 1(a)] or between the needle and the barrel [Figure 1(b)] as a cartridge. The cartridge bed can be packed or coated to provide selective and suitable sampling conditions. Any sorbent material such as silica-based material (C2, C8, C18, SCX), restricted access material (RAM), carbon, polystyrene-divinylbenzene copolymer (PS-DVB) or molecular imprinted polymers (MIPs) can be used. Also, a liquid polymer coating can be provided. MEPS is commercially available from SGE (Melbourne, Australia; www.sge.com). Additionally, a solution kit for MEPS and the CTC autosampler is available from SciSEP (Chelmsford, UK).
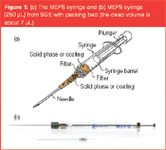
Figure 1
How it works: The plasma sample (10–250 μL) is first diluted with water (1:4) and centrifuged for 1 min; for whole blood, the dilution is 20-fold. The plasma or blood sample is drawn through the syringe by an autosampler (which pumps the sample up and down) through the sorbent (Figure 2). The sample can be pumped once or more if preconcentration of the analytes is required. When the sample has passed through the solid support, the analytes have been adsorbed to the solid phase, which is then washed once with water (50 μL) to remove proteins and other interfering material. The analytes are then eluted with an organic solvent, such as methanol, or the LC mobile phase (20–50 μL) directly into the instrument's injector GC or LC. The process is fully automated. The MEPS sorbent can be used for more than 100 extractions before being discarded. We observed that a conditioning step is not necessary as in SPE. This may be because the smaller amount of the sorbent or because the pumping of the sample through the sorbent more than one time. In addition the connection of MEPS to GC was made using large volume injection (30–100 μL). We did not observe any deterioration effect on the GC performance, after injection of many hundreds of samples on the same column as a result of the non-drying of the sorbent after washing. This may be because the dead volume is less than 7 μL. Additionally, we added to our CTC-macro that the CTC can elute 5 μL from elution solution to the waste (as drying step) before injection but we did not observe any effect on the performance with this step.
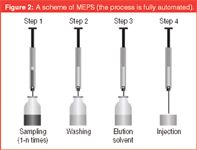
Figure 2
Washing and Elution Solutions
Washing solution: The purpose of the washing step is to remove unwanted weakly retained interferences. A typical solution may contain less organic solvent than the final elution solution. The solvent percentage and the pH are important factors in decreasing the leakage of analytes during the washing process. It has been shown that the analyte leakage increases as the washing volume or solvent percentage increases.4,24 Using 10% methanol in water reduced the recovery by about 10% compared with water alone. Leakage increased with the methanol percentage (Figure 3). For basic drugs, the optimal washing solution contains methanol (≤10%) in water containing 0.1% formic acid.
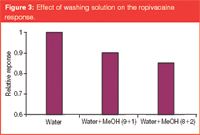
Figure 3
Elution solution: A pure or high solvent percentage (≥60%) is a typical elution solution. Furthermore, the pH is an important factor (control charged/uncharged analyte) for a high recovery. The best possible elution solvent should elute the analyte using the smallest volume possible. The analyte response increases as the solvent percentage and elution volume increase.24 Figure 4 shows the extraction of basic drug (ropivacaine) from plasma samples: It was found that the optimal recovery was obtained when 0.25% ammonium hydroxide (pH > 10) was added to an elution solution containing 95% methanol and 5% water.3–5
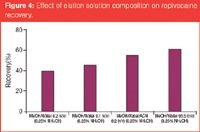
Figure 4
Study of carryover: Carryover is one of the common problems with automated systems. It is a limiting step for trace analysis, giving poor accuracy and precision during method validation. Carryover can also reduce the dynamic range of MS instruments due to its limitation in decreasing the limit of detection (LOD). It was, therefore, important to study carryover for MEPS. First of all, carryover is a compound-dependent phenomenon and can be caused not only by MEPS but also by analyte adsorption to an autosampler or LC system or MS interface.
The small quantity of phase in the MEPS can easily and effectively be washed out between samples to reduce the possibility of carryover. With the automation of MEPS, washing can occur while the previous sample is running. Carryover decreased to less than 0.1% when the sorbent was washed at least five times with elution solution and washing solution between extractions. Figure 5 shows how the carryover decreased to 0.02% for bupivacaine (2000 nM) after four washes with the elution solution (60% methanol in water).

Figure 5
Syringe-to-syringe variation: The reproducibility measurements of MEPS showed good relative standard deviation (RSD) % values concerning analyte recovery for different analytes and different matrices. The syringe-to-syringe variations were also tested. Table 2 shows the variations between three different syringes using AZD3409 (AstraZeneca's new drug candidate). AZD3409 is a novel oral protein prenyl transferase inhibiting both farnesyl transferase and the geranylgeranyl transferase-1. MEPS sorbent was C8, washing solution (100 μL) was 0.1% formic acid in water and elution solution (40 μL) was 95% methanol in water. Using different concentrations at three levels, the accuracy and the precision are similar for all the syringes studied.
Table 1: Summary for MEPS applications of drugs and metolites from biological samples.
Drugs and Metabolites from Biological Samples
MEPS has been used for the extraction of many drugs and metabolites from biological samples. The drugs extracted by MEPS are summarized in Table 1.

Table 2: Accuracy and precision at various concentrations for different MEPS (C8) syringes using AZD3409.
Extraction of local anesthetics from plasma: MEPS was used for the extraction of the amide-type local anesthetics lidocaine, ropivacaine, mepivacaine, prilocaine and bupivacaine and some of their metabolites from plasma samples were reported.3–5,9,10,12 Figure 8 shows the standard curves of the studied compounds in human plasma using MEPS-GC–MS.

Figure 6
MIP-MEPS on-line with LC–MS–MS for quantification of ropivacaine in plasma was also investigated.10 Bupivacaine-imprinted polymer was used. The calibration range was 2–2000 nM and the extraction recovery was 60%. The results showed close correlations (r2 > 0.999) for all runs. Accuracy, stated as a percentage variation from the nominal concentration values, ranged from –6–3%, while precision, stated as the relative standard deviation (RSD), at three different concentrations (QC samples) was consistently about 3–10%. The limit of quantification was 2 nM.
Extraction of anticancer agents: Busulphan and cyclophosphamide (alkylating agents and the most widely used anticancer drugs) were extracted and determined by MEPS-LC–MS–MS.18,19 The validation showed that selectivity, accuracy and precision were satisfactory. The busulphan bioanalytical method using MEPS-LC–MS gives both accuracy and precision within the range of therapeutically relevant levels (5–2500 ng/mL). It also reduces the sample preparation time for busulphan [less than 1 min per sample compared with 40 min using liquid–liquid extraction (LLE)], which is of great importance in adjusting the busulphan dose in clinical settings. Figure 7 shows a mass chromatogram for patient blank and patient plasma samples.
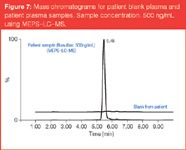
Figure 7
The sample concentration was 500 ng/mL using MEPS-LC–MS.
MEPS was used on-line, followed by liquid chromatography with liquid chromatography tandem mass spectrometry (LC–MS–MS), for the quantification of cyclophosphamide in plasma samples.19 The new method reduced sample handling and analysis time several-fold compared with LC with UV detection. The limit of detection (LOD) was 0.005 μg/mL and the lower limit of quantification (LLOQ) was 0.5 μg/mL. The accuracy of the quality control (QC) samples ranged from 95–106%. The inter-day variation was in the range 5–9%, while the intra-day variation was between 1 and 5%. The calibration curve in plasma was constructed in the concentration range 0.5–150 μg/mL. The regression correlation coefficient (r2) was ≥0.99 for all runs. The LOD improved 100-fold using MEPS-LC–MS–MS (0.005 μg/mL) compared with LLE-LC-UV (0.5 μg/mL). The method was employed for the quantification of cyclophosphamide in human plasma samples for more than 170 patient samples. Table 3 gives a comparison between MEPS and several published methods for the determination of cyclophosphamide in plasma samples.

Table 3: Comparison between MEPS and other methods for determination of cyclophosphamide in plasma samples.
Roscovitine (2-(R)-(1-ethyl-2-hydroxyethylamino)-6-benzylamino-9-isopropylpurine) was also extracted from plasma and urine samples using MEPS and has recently been regarded as a possible new chemopreventive and chemotherapeutic approach. The drug selectively inhibits cyclin-dependent kinases (Cdks), which are enzymes that play a crucial role in cell cycle regulation and apoptosis. The sampling sorbent was polystyrene (ENV+, 1 mg). The accuracy values of quality control (QC) samples were between 96% and 115%, and the maximum deviation for precision (RSD) was 11.4%.
Figure 8
MEPS was used to extract a new candidate drug AZD3409 from plasma samples. It is highly unstable: its metabolism involves conversion to a thiol ester intermediate and then intracellularly to a thiol acid active moiety (Figure 8). Due to the instability of AZD3409, MEPS was used as an on-line and fast sample-preparation method, followed by liquid chromatography tandem mass spectrometry (LC–MS–MS) for the quantification of this compound in plasma samples. High accuracy and precision values were obtained and the method was used for the analysis of rat and dog plasma samples.20 Figure 9 shows a mass chromatogram obtained from MEPS extraction of spiked rat plasma of a QC sample (5.4 μM AZD3409) and blank rat plasma sample.

Figure 9
Extraction of β-blocker drugs from plasma: Acebutolol and metoprolol were extracted from plasma using MEPS15 on-line with high-performance liquid chromatography and tandem mass spectrometry (HPLC–MS–MS). The LLOQs for acebutolol and metoprolol were set to 1.0 ng/mL. The accuracy for QC samples varied from 94–104%. The precision (RSD%) was in the range 9.5–12%.
Ropivacaine and its metabolites in urine samples: The extraction of ropivacaine and its metabolites from urine samples using MEPS was reported.11 The MEPS sorbent used was polystyrene polymer and the analysis technique used was LC–MS–MS. The sample volume was 50 μL (pumped three times) and was washed with 50 μL water. The elution solution was 0.2% ammonium hydroxide in methanol (20 μL). The LLOQ was 5.0 nmol/L. The calibration curves were obtained within the concentration range 5–2000 nmol/L in urine. The regression correlation coefficients for urine samples were 0.999 for all runs. The extraction degree was 40–60%. The between-batch accuracy and precision values of the ropivacaine and its metabolites were determined from six replicates of QC samples at three different concentrations in human urine. The mean accuracy reported for the QC samples was in the range 99–115% and the precision was in the range 1.9–11%. The accuracy and precision of ropivacaine and its metabolites in urine samples are summarized in Table 4.

Table 4: The accuracy and precision of ropivacaine and its metabolites in urine samples.
4-OH-2,6-xylidine and its conjugates in human urine: 4-OH-2,6-xylidine is a major metabolite of lidocaine. 4-OH-XYL has been found to be stable under highly acidic conditions (pH ≤ 1) and highly unstable in neutral and alkaline solutions. Our investigation showed that 4-OH-XYL is stable for at least 24 h in a refrigerator in 0.1 M HCl (pH ≈ 1). At pH 9.1, more than 40% decomposition of 4-OH-XYL occurs within 6 h at room temperature compared with 20% at pH 2.0. Therefore, our approach was to use acid hydrolysis of 4-OH-XYL conjugates and on-line sample preparation for the quantification of 4-OH-XYL in human urine. The validation of the method was performed in the range 17–8700 nmol/L. The inter-day accuracy and precision values were determined from six replicates of QC samples at three concentrations in human urine. The method was applied to human urine samples from a clinical study.13
β-blocker drugs in urine samples: Determination of the β-blockers acebutolol and metoprolol in urine15 was performed using MEPS as a sample preparation method, on-line with HPLC–MS–MS. The LLOQ for acebutolol and metoprolol were set to 1.0 ng/mL. The accuracy of QC samples varied by ±10% and precision (RSD) had a deviation of 1.4–12%. The calibration curve was obtained in the concentration range 1.0–100 ng/mL in both plasma and urine. The regression correlation coefficients (r2) for plasma and urine samples were 0.999 for all runs.
Cocaine and its metabolites in urine samples: MEPS has been evaluated for fast screening of drugs of abuse using MS detection. Several sorbents such as C8, ENV+, Oasis MCX and Clean Screen DAU (United Technologies, Bristol, Pennsylvania, USA) were used. In this study the focus was on fast extraction and preconcentration of the drug and metabolites with rapid analysis using a time-of-flight (TOF) mass spectrometer as the detector with direct analysis in real time (DART) source. The analysis time was less than one minute. This study has demonstrated that the combination of MEPS with DART–TOF-MS can be a very useful tool for screening drugs of abuse in a biological matrix. Furthermore, the study attempted to demonstrate that it is possible to quantify the analyte of interest using a DART source when an internal standard (IS) is used.23
Dopamine, serotonin and methadone in urine samples: Dopamine, serotonin (5-hydroxytryptamine) and methadone were extracted from human urine samples, preconcentrated using MEPS and determined by LC–MS–MS and GC–MS.16,17 The new method is fully automated, inexpensive and rapid compared with published methods. The calibration range was 50–4000 μg/L. The MEPS sorbent was silica-C8, which can be used more than 300 times. The extraction recovery was about 50% and the accuracy of MEPS–LC–MS–MS was 100–101% for dopamine and 99–100% for 5HT. The inter-day precision (RSD%, n = 3) was 6.0–7.7% for dopamine and 6.1–11% for 5HT. MEPS reduced the handling time 10-fold compared with a published method. The LOQ and LOD for dopamine and serotonin were 50 μg/L and 1.0 μg/L, respectively. Carryover was tested by injecting a blank after the highest standard concentration and was found to be in the approximate range of 0.1–0.2%. MEPS has an accuracy and precision comparable to those of the published methods. In addition, it improved the limit of detection two-fold and reduced the extraction time by approximately 12-fold.
A method for the simultaneous analysis of methadone in urine samples by microextraction by packed sorbent on-line with GC–MS (MEPS-GC–MS) is described.17 The intra-assay precision (RSD%) of the method was about 11–14% (n = 6). It was 11–15% for methadone in urine samples (n = 18). Accuracy varied from 89% to 109% for intra-assay (n = 6) and from 97% to 107% for inter-assay (n = 18). Table 5 shows a comparison of MEPS with other extraction methods that were published for the extraction of methadone.

Table 5: Comparison of accuracy, precision, limit of detection, and extraction time of methadone between MEPS and earlier published methods.
Whole blood samples: The quantitative determination of drugs and metabolites in blood has become more and more important in forensic and clinical toxicology. The extraction of drugs from human blood and mouse blood samples using MEPS on-line with LC–MS–MS has been performed.21,22
Local anesthetics in human blood: The first paper illustrates the extraction of local anesthetics (lidocaine, ropivacaine, bupivacaine) from human blood samples directly by MEPS-LC–MS–MS. The blood samples were diluted with 0.1% HCOOH before MEPS handling. The LLOQ was set to 10.0 nM for all the studied analytes. The validation of the method showed that the accuracy of the QC samples ranged from 85–97%. The inter-day precision of the studied analytes was in the range 1–5%. The calibration curve in plasma was constructed in the concentration range 10–10000 nM. The regression correlation coefficient (r) was >0.995 for all runs (n = 3). Figure 10 shows a mass chromatogram of blank and spiked blood samples for lidocaine, ropivacaine and bupivacaine (50 nM each).

Figure 10
Cyclophosphamide in mouse blood: The second paper illustrates the extraction of the anticancer drug cyclophosphamide in whole mouse blood directly by MEPS-LC–MS–MS. 20 μL of mouse blood was mixed with 80 μL of the anticoagulant agent (EDTA). The blood samples were diluted five-fold with 0.1% HCOOH before MEPS handling. The LLOQ was set to 0.1 μg/mL. The accuracy of the QC samples ranged from 96–114%. The inter-day variation was in the range 2–9%, while the intra-day variation was between 5–9%. The calibration curve in plasma was constructed in the concentration range 0.1–100 μg/mL. The regression correlation coefficient (r2) values were over 0.99 for all runs (n = 3).

Keynotes
Conclusions
The technique of MEPS is apparently very attractive — it can cope with much smaller samples than full-scale SPE (as small as 10 μL). This approach to sample preparation is very promising for many reasons: (1) it is easy-to-use, (2) it is a fully automated on-line procedure, (3) it is rapid and (4) the cost of analysis is minimal compared with conventional SPE. MEPS can also be easily automated and uses smaller volumes for sample, solvent and dead volume in the system. Other significant advantages include the speed and the simplicity of the sample preparation process. The key aspect of MEPS is that the solvent volume used for the elution of the analytes is of a suitable order of magnitude to be injected directly into GC or LC systems. MEPS significantly reduces the volume of solvent and sample needed.
Future work should be focused on the extraction of more drugs and metabolites. A broad range of applications in different areas, such as environmental and food analysis, will be needed. MEPS could be used for on-site environmental analysis. More selective sorbents and the use of antibodies for more selective extraction will be investigated. MEPS is adaptable to other analytical techniques, including immunoassay and off-line analysis by nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy and other methods.
Mohamed Abdel-Rehim currently works at AstraZeneca (Department of Clinical Pharmacology & DMPK), Södertälje, Sweden. He obtained his PhD in Pharmaceutical Science from Uppsala University, Sweden and is now adjunct professor in the Department of Chemistry and Biomedical Sciences at Karlstad University, Sweden. Additionally, he is a visiting professor at the National Research Center of Egypt. Abdel-Rehim has published over 190 publications (scientific papers, proceedings, AstraZeneca internal research reports, patent, etc.). The main focus of his research is the invention of highly automated and integrated instrumentation for the isolation of drugs and metabolites from complex matrices and the subsequent separation, identification and determination of these analytes using GC–MS and LC–MS–MS in addition to preparation of new sorbents such as monolithic phases for bioanalysis applications.
References
1. M. Abdel-Rehim, AstraZeneca Application, Current Patents Gazette, week 0310, WO 03019149, 77 (2003).
2. M. Abdel-Rehim, PCT, World Intellectual Property Organization, International Publication Number: WO 03/019149 A1, International Publication Date: 6 March 2003.
3. M. Abdel-Rehim, J. Chromatogr. B, 801, 317–321 (2004).
4. M. Abdel-Rehim, Z. Altun and L. Blomberg, J. Mass Spectr., 39, 1488–1493 (2004)
5. Z. Altun, M. Abdel-Rehim and L.G. Blomberg, J. Chromatogr. B, 813, 129–135 (2004).
6. M. Vita et al., J. Chromatogr. B, 817, 303–307 (2005).
7. M. Vita et al., J. Chromatogr. B, 821, 75–80 (2005).
8. M. Abdel-Rehim et al., Anal. Chim. Acta., 539, 35–39 (2005).
9. Z. Altun et al., J. Liq. Chromatogr. & Relat. Technol., 29, 829–839 (2006).
10. M. Abdel-Rehim et al., J. Liq. Chromatogr. & Relat. Technol., 29, 1725–1736 (2006).
11. M. Abdel-Rehim, M. Dahlgren and L. Blomberg, J. Sep. Sci., 29, 1658–1661 (2006).
12. M. Abdel-Rehim et al., J. Liq. Chromatogr. & Relat. Technol., 29, 2537–2544 (2006).
13. M. Abdel-Rehim et al., J. Liq. Chromatogr. & Relat. Technol., 29, 2413–2424 (2006).
14. A. El-Beqqali, A. Kussak and M. Abdel-Rehim, J. Chromatogr. A, 1114, 234–238 (2006)
15. A. El-Beqqali et al., J. Liq. Chromatogr. & Relat. Technol., 30, 575–586 (2007).
16. A. El-Beqqali, A. Kussak and M. Abdel-Rehim, J. Sep. Sci., 30, 421–424 (2007).
17. A. El-Beqqali and M. Abdel-Rehim, J. Sep. Sci., 30, 2501–2505 (2007).
18. M. Abdel-Rehim et al., J. Liq. Chromatogr. & Relat. Technol., 30, 3029–3041 (2007).
19. R. Said et al., J. Liq. Chromatogr. & Relat. Technol., 31, 683–694 (2008).
20. M. Abdel-Rehim et al., J. Chromatogr. Sci., 46(6), 518–523 (2008).
21. M. Kamel et al., J. Liq. Chromatogr. & Relat. Technol., Submitted.
22. M. Kamel et al., J. Chromatogr. A, In manuscript.
23. E. Jagerdeo and M. Abdel-Rehim, Submitted to JAMS.
24. Z. Altun and M. Abdel-Rehim, Anal. Chemica Acta., 630, 116–123 (2008).
25. N. Sadagopan et al., J. Chromatogr. B, 759, 277–284. (2001).
26. M.E. De Jonge et al., J. Mass Spectr., 39, 262–271 (2004).
27. A. Barbieri et al., Rapid Commun. Mass Spectrom., 20, 1889–1893 (2006)
28. S.W. Myung et al., Analyst, 124, 1283–1286 (1999).
29. H. He et al., J. Chromatogr. B, 814, 385–391 (2005).
30. O. Quintela et al., J. Chromatogr. B, 834, 188–194 (2006).
Best of the Week: Food Analysis, Chemical Migration in Plastic Bottles, STEM Researcher of the Year
December 20th 2024Top articles published this week include the launch of our “From Lab to Table” content series, a Q&A interview about using liquid chromatography–high-resolution mass spectrometry (LC–HRMS) to assess chemical hazards in plastic bottles, and a piece recognizing Brett Paull for being named Tasmanian STEM Researcher of the Year.
Using LC-MS/MS to Measure Testosterone in Dried Blood Spots
December 19th 2024Testosterone measurements are typically performed using serum or plasma, but this presents several logistical challenges, especially for sample collection, storage, and transport. In a recently published article, Yehudah Gruenstein of the University of Miami explored key insights gained from dried blood spot assay validation for testosterone measurement.
Determination of Pharmaceuticals by Capillary HPLC-MS/MS (Dec 2024)
December 19th 2024This application note demonstrates the use of a compact portable capillary liquid chromatograph, the Axcend Focus LC, coupled to an Agilent Ultivo triple quadrupole mass spectrometer for quantitative analysis of pharmaceutical drugs in model aqueous samples.