- The Application Notebook-10-02-2010
- Volume 0
- Issue 0
Confirmation and Quantification of Cocaine and Major Metabolites in Urine Using the ISQ Single Quadrupole GC-MS
Thermo Fisher Scientific Application Note
Matthew Lambing, Eric Phillips and Trisa Robarge, Thermo Fisher Scientific Inc., Austin, Texas, USA.
Cocaine is a central nervous system stimulant derived from Erythroxylon coca. It is metabolized in vivo resulting in the formation of ecgonine methyl ester, norcocaine and benzoylecgonine. Cocaethylene is a substance formed when cocaine and ethanol are coadministered.1 A forensic toxicology method for the confirmation and quantification of ecgonine methyl ester (EME), benzoylecgonine (BE), cocaine (COC) and cocaethylene (CE) in human urine was developed using the Thermo Scientific ISQ Single Quadrupole GC–MS system.
Methods
Each 2 mL validation sample batch included a matrix-matched single point calibrator (at 150 ng/mL), quality control samples set to contain each target compound at 40% and 125% of the calibrator (60 ng/mL and 187.5 ng/mL respectively) and a negative control (blank urine with internal standard only). Thermo Scientific HyperSep Verify-CX solid-phase extraction columns were used for sample extraction and derivatized with hexafluoroisopropanol (HFIP) and pentafluoropropionic acid (PFPA or PFAA).
The ISQ mass spectrometer system was operated in selected ion monitoring mode, collecting 3 ions for each target compound and 2 ions for each deuterated internal standard (Table 1). A Thermo Scientific AS3000 II autosampler and a Thermo Scientific TRACE GC Ultra gas chromatograph, equipped with a split/splitless injection port, provided sample introduction and separation. A 15 m × 0.25 mm i.d. × 0.25 µm film thickness Thermo Scientific TraceGOLD TG-5MS analytical column was used to enhance separation of the target cocaine class compounds from each other and from matrix components. Thermo Scientific ToxLab Forms software automated the acquisition and processing of all data, including quantification and ion ratio confirmation calculations. For precision analyses, a coefficient of variation of <10% of the average calculated quality control amounts were required for each analyte and interday percent differences of calculated amounts were also less than 10%.
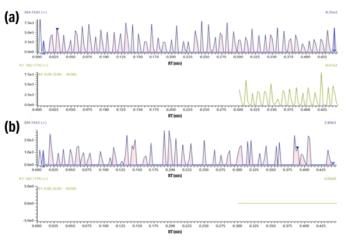
Figure 1: Separation and detection of four drug standards and six inorganic anion standards.
Results
- Assay linearity ranged from 15 ng/mL to 12500 ng/mL for BE, EME and CE, and 15 ng/mL to 5000 ng/mL for cocaine.
- Limits of detection and quantification of 15ng/mL using a 2 mL sample size.
- Intra- and inter-day precision of <10% CV at the quality control levels of 60 ng/mL and 187.5 ng/mL.
- Correlation coefficient (R2) better than 0.9990 for cocaine, benzoylecgonine, ecgonine methyl ester and cocaethylene based on a one point calibration.
- Pseudoephedrine at a concentration of 20000 ng/mL showed interference with EME at the 40% and 125% QC levels.
- Norcocaine at 10000 ng/mL demonstrated no interference with any analyte tested, but limited co-elution was observed with cocaethylene. Relative retention time to CE = 1.005.
Conclusion
The assay described offers broad linearity to cover a wide range of cocaine and major metabolite concentrations, thus, reducing the need for dilutions or repeat extractions.
Excellent precision was also demonstrated around the 150 ng/mL cutoff, with CV measurements of 10% or less over the study. Limits of detection and quantification at 15 ng/mL ensure sensitive performance for retest and directed assay samples. The methodology described offers a means for a forensic toxicology laboratory to confirm and quantify cocaine, benzoylecgonine, ecgonine methyl ester and cocaethylene in human urine.
Reference
1. Disposition of Toxic Drugs and Chemicals in Man, Eighth Edition. Randall C. Baselt, Biomedical Publications, 2008.
All trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries.
Thermo Fisher Scientific
2215 Grand Avenue Parkway, Austin, Texas 78728, USA
tel. +1 800 532 4752 fax +1 561 688 8731
E-mail:
Website:
Articles in this issue
about 15 years ago
Light Scattering for the Masses Heparin Characterizationabout 15 years ago
Is There Really a Difference Between Flash and HPLC for LC Purification?about 15 years ago
Peptide Mapping of a Monoclonal Antibody Using Agilent Poroshell 120about 15 years ago
Extend UHPLC Column Lifetime with KrudKatcher Ultra In-line Filtersabout 15 years ago
Making Non-Electrochemically Active Compounds Electrochemically ActiveNewsletter
Join the global community of analytical scientists who trust LCGC for insights on the latest techniques, trends, and expert solutions in chromatography.