The Beginnings of Gas Adsorption Chromatography 60 Years Ago
LCGC North America
Work done 60 years ago at the University of Innsbruck (Austria), representing the start of gas chromatography, is outlined in this month's column.
Usually the activities of A.J.P. Martin and A.T. James in 1950–1952 at the British National Institute of Medical Research, in London, are considered as the start of gas chromatography (GC). After a preliminary report presented at the 290th Meeting of the Biochemical Society, on October 20, 1950 (1), their results were elaborated in a fundamental paper submitted on June 5, 1951, to Biochemical Journal, and published in its March 1952 issue (2). However, this paper only represented the start of gas–liquid partition chromatography: gas adsorption chromatography, based upon selective adsorption–desorption steps in a gas stream, has its origins in the decade preceding the work of James and Martin. In fact, industrial separation processes based upon the adsorption–desorption of gases and vapors have been developed even earlier, although at that time these were not considered as "chromatography:" it was only recognized much later that these processes also represent different versions of chromatography.

Leslie S. Ettre
Restricting ourselves to laboratory investigations we can point to a few researchers who, in the first part of the 1940s, developed separation techniques based upon some version of gas adsorption chromatography: these are Gerhard Hesse, at the University of Marburg/Lahn, in Germany (3,4); N.C. Turner, in association with the Burrell Corporation, in Pittsburgh, Pennsylvania (5,6); and Stig Claesson, at the University of Uppsala, Sweden (7). However, their investigations were more or less isolated, without any followup and did not contribute to the overall evolution of chromatography. On the other hand, the activities of Professor Erika Cremer and her students at the University of Innsbruck, Austria, in the years following the Second World War, represented the true start of their continuous involvement in GC. The first of these investigations was finished 60 years ago, in May 1947, as part of the Ph.D. thesis work of Fritz Prior. Thus, it is time to dig out these long forgotten studies from oblivion: they represent an integer, and important, part of the long evolution of GC.

Background
Erika Cremer (1900–1996), a native of Munich, Germany, and a descendant of generations of university professors, studied at the University of Berlin, receiving the best education a chemistry student could obtain at that time anywhere in the world. However, there was still a serious prejudice against female scientists, particularly in Germany: the opinion expressed by Emperor Wilhelm II, that women should deal only with the three K's — Kinder, Kirche, Küche (children, church, kitchen) — was still very much in effect. Thus, in spite of her excellent background and superb Ph.D. thesis work, she could only find relatively minor, subordinate positions in research laboratories. However, in this way she had the opportunity to become associated with the biggest names in chemical research: Fritz Haber, Georg von Hevesy, Michael Polanyi, and Otto Hahn. Finally, when the war broke out and many of the university teachers were enlisted in the army, there opened up for her the possibility to be associated with a university. In fact, she even was able to select among a few schools. She decided for the University of Innsbruck (at that time Austria was part of Germany), because of her love of the mountains, and the closeness to Munich, the city of her birth. She was, however, warned that at war's end, "when the boys return," she might have to give up his position (8).
Cremer started in 1940 as a Privat Dozent (equal to an associate professor rank) at the Institute of Physical Chemistry of Innsbruck University, and was involved in both teaching and in research, dealing with the catalytic hydrogenation of acetylene to ethylene. In the course of these activities, a method for the reliable quantitative analysis of acetylene and ethylene in their mixture would have been needed. Being a physico-chemist she was considering to utilize selective adsorption–desorption, similar to the way Peters and coworkers at the Kaiser Wilhelm Institut für Kohlenforschung, in Mülheim/Ruhr, separated in the 1930s noble gases (9,10). Some preliminary investigation was carried out in the thesis work of Achim Kunte (11), who also built a thermal-conductivity detector; however, these measurements have indicated no clear difference in the adsorption isotherms of these two compounds, thus, precluding the possibility of separation by the then existing (nonchromatographic) techniques.
In 1943, Gerhard Hesse published a book on the use of adsorption in which he dealt in details with classical (liquid) adsorption chromatography originated by Tswett (12). Reading this book Cremer was particularly interested in Hesse's explanation how elution chromatography works and — as a physical chemist — she related the migration of the sample molecules along the (adsorption) column to their heats of adsorption. She actually selected this subject as the thesis work of Reingard Knöpfler, a new graduate student at the Institute (13). Her work was carried out under great difficulties because of the damages by the air raids on Innsbruck and the university buildings. The Institute of Physical Chemistry was mostly destroyed, had to be moved to temporary locations in Wattens, a town about eight miles east of Innsbruck, and travel was possible only by bicycle from Innsbruck. Knöpfler finally finished her work only in January 1945.
Cremer also considered theoretically this question and derived an equation describing the difference between the heats of adsorption (ΔH) of two substances as a function of the distances s1 and s2 their molecules travel along the column in a given time:
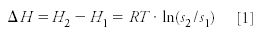
where T is the (absolute) temperature and R is the gas constant. Furthermore, she also speculated that if a gas and not a liquid is used as the mobile phase, the time the individual substances are retained by the column (corresponding to the adjusted retention times, according to our present nomenclature) could be substituted for the distances s1 and s2. Eventually she summarized these theoretical considerations in a short paper entitled "The Migration Speed of Zones in the Chromatographic Analysis," and submitted it in November 1944 to the editorial offices of the journal Naturwissenschaften (then in Vienna). Her paper was soon accepted; she received the proofs and returned them on February 6, 1945 (she kept a copy until the end of her life). However, soon the printing office of the journal and, thus, all the materials for the next issues, were destroyed in an air raid. Thus, Cremer's paper could not be published at that time. (It was finally published 30 years later, as a historical document, together with her remarks about its origin [14]).
The validity of equation 1 in liquid adsorption chromatography was investigated in a limited extent by Reingard Knöpfler in her thesis work. Cremer also planned some laboratory work to test the possibility of using a gas as the mobile phase in elution chromatography (quite revolutionary idea at that time!) and to see whether the relationship expressed by equation 1 also would be valid for that case. However, there was no possibility for this during the last months of the war.
(We shall mention here that during the war and even for a few years after it, British journals were unavailable in Germany and Austria. Thus, the development of partition chromatography by Martin and Synge, in 1941, and the suggestion in their paper [15] on the possible use of a gas as the mobile phase was unknown to them.)
The First GC Investigations
By the end of the war Austria became again independent, but was divided into four occupation zones (Innsbruck was now in the French zone), and this created a number of restrictions in movements and communications. Slowly rebuilding started and finally the university could reopen in the fall of 1945, although still in temporary quarters and under primitive conditions, and the students returned only slowly. The fact that now Cremer was legally a German citizen created a temporary problem. However, it was soon solved and because the former institute director left Innsbruck, she was appointed as its acting head.

Figure 1
As narrated by Cremer (14), in November 1945, a young man named Fritz Prior (born 1921) came to her with the aim to work for the Ph.D. degree (Figure 1). Prior just started as a teacher at a high school outside Innsbruck: what was most important, that building was undamaged and had a physics laboratory. As the thesis subject Cremer selected the investigations she planned to carry out before the destruction of the university institute: to see whether GC works, and whether the theoretically derived relationships are also valid in gas adsorption chromatography. Prior's high school lab had most of the needed basic hardware — glass tubes, stopcocks, a Kipp hydrogen generator (producing hydrogen from the reaction of zinc and hydrochloric acid), and an ancient galvanometer. Kunte's thermal-conductivity detector was destroyed with the university building, but Cremer still had a piece of the wire used in it as the sensing element, permitting to build a new detector. Thus, they were able to assemble the necessary setup shown in Figure 2. Hydrogen, produced in the Kipp generator and purified in a number of steps, was used as the carrier gas. The gas samples were introduced using a gas buret, with a number of stopcocks; gas mixtures could be prepared in the buret before introduction. A 1-cm diameter glass U-tube served as the column; its temperature was kept constant by a Dewar flask. Both silica gel (Kieselgel) and active carbon served as the adsorbent; the length of the packing in the column was about 20 cm. The homemade thermal-conductivity cell also was thermostated. Its construction was more primitive than the readers of our journal are familiar with: there was only one single cell. The Wheatstone bridge also was self-constructed. At that time (and for about 10 more years) potentiometric recorders well known in the Unites States were not available in Europe. The detector had to be connected to Prior's ancient galvanometer, its deflection recorded and then plotted manually against time. (This is the reason for the many dots in the chromatograms shown in Figures 3–6).
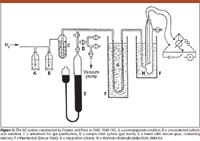
Figure 2
As mentioned the primary goal of Prior's investigation was to prove the correctness of Cremer's assumed relationship expressing the difference of the heats of adsorption of two substances (ΔH) at a given temperature (T), as a function of their adjusted retention times (t'R):
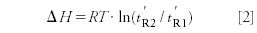
Also, he tested the validity of the relationship of the heats of adsorption (H) of a single substance from chromatographic measurements at two temperatures:
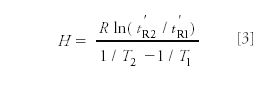
Naturally, however, he first had to demonstrate that chromatography with a gas as the mobile phase works, and that gas mixtures can be separated by gas adsorption chromatography. Figures 3–5 show some of the chromatograms included in Prior's Ph.D. dissertation (16). He also investigated a number of important fundamental questions pertaining to GC: the influence of temperature and the carrier gas flow rate on separation, the difference between the total elution time and what we call today the adjusted retention time, that is, the time the sample molecules are actually retarded by the adsorbent. He also investigated the reproducibility of the retention times and of the measured values of H and ΔH, and his thesis report contains a number of data and plots.
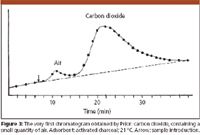
Figure 3
For a present-day chromatographer Prior's chromatograms look fairly primitive: the peaks are very broad and asymmetrical, the baseline is drifting and resolution is not complete. However, we cannot judge them with our present knowledge. Many questions were unsolved: the adsorbent was not uniform; as we have seen the detector had a primitive construction; the introduced sample volumes were too high, and the criteria of optimum operation were not yet established. The chromatograms from the mid-1950s, obtained by gas–liquid partition chromatography, and published in the papers of early researchers, also show similar shortcomings; we have learned only gradually how to optimize the conditions and how to achieve the best results. The importance of Prior's work is that his chromatograms were the first showing that GC is feasible and that he started to establish some of the rules and interpretations that then slowly became self-evident.
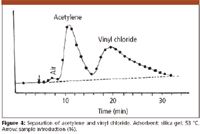
Figure 4
Prior finished his work in May 1947. At the end of his thesis, Prior mentioned that the new technique also could be used successfully for quantitative analysis, that is, for the determination of the composition of gas mixtures, and that much smaller sample volumes (below 1 mL) also could be analyzed successfully. The exploration of these aspects was then the main subject of the thesis work of Roland Müller, a new graduate student joining the Institute in the fall of 1947: He concentrated on the analytical aspects, investigating the possibility of qualitative and quantitative analysis, and clearing up some of the shortcomings in Prior's work. The improvements can be seen if we compare the chromatogram of Müller shown in Figure 6 with Prior's chromatograms (Figures 3–5), and the difference in the emphasis of the two works is evident if we compare the title of Prior's thesis (16) with that of Müller finished in May 1950 (17). It is noteworthy to mention here that Müller's work also introduced a number of new terms which later became commonly used in GC, for example, the peak width at half height and the use of its product with the peak height to approximate the peak area.
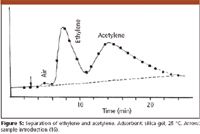
Figure 5
Belated Recognition
When Prior finished his thesis in the spring of 1947, publication of scientific papers and even organization of scientific meetings was practically impossible in Austria (and also in Germany). Thus, while the full text of his thesis went through the usual internal review by two professors and then was deposited at the University archives, it was known only to a limited number of people.
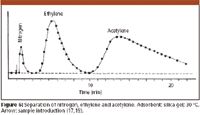
Figure 6
One of the first scientific meetings in Austria was held in May 1949 in Linz, and there Cremer presented a brief report summarizing Prior's results, but only the abstract of the lecture was published (18). Then in May 1950 she was able to participate at the meeting of the Bunsengesellschaft (the German physico-chemical society) held in Marburg/Lahn. At this meeting Hesse presented a paper on the adsorption process in a (liquid) chromatographic column, and in the discussion Cremer made a 130-word comment, mentioning the work on gas adsorption chromatography done in Innsbruck, and showing a chromatogram from Müller's thesis (Figure 6 here). Hesse's paper was published in the January 1951 issue of the Zeitschrift für Elektrochemie (the journal of the Bunsengesellschaft), together with Cremer's remark (19).
Finally Cremer had the opportunity in July 1950 to present a full paper at a major international symposium: at the First Microchemical Congress held in Graz, Austria. However, bad luck followed her even to this meeting. At 3:45 PM on July 5 — the exact time when she presented her paper — the temperature in Graz reached the highest ever recorded: 37.2 °C (99 °F), and the session was held at the university auditorium, with closed windows and no air conditioning, resembling more a furnace than a lecture room. Professor Cremer remembered even toward the end of her life, that only a few brave microchemists dared to be there: most of the meeting participants were at the city's municipal swimming pool, or in the Stadtpark (city park) of Graz, trying to find relief in the water or under the ancient trees.
By the time of the Graz meeting Cremer already submitted a manuscript (on her work with Prior) to the Zeitschrift für Elektrochemie (20) that was then published in its January 1951 issue. This was followed on August 25, 1950, by another manuscript, now submitted to Mikrochemie/Mikrochimica Acta, the journal of the Austrian Microchemical Society (21), and finally, by a third paper submitted on January 19, 1951, again to the Zeitschrift für Elektrochemie (22). These two papers were published later in 1951.
It belongs to our story that Cremer's presentations received a negative response or no response at all. In retrospect, we may explain this by the fact that she spoke to the wrong people, at the wrong meeting, and on the wrong subject. In Austria, analytical chemistry by tradition meant mainly classical microchemistry of the Pregl–Emich type, and the microchemists were certainly not interested in the analysis and separation of gases. Also, the German physico-chemists assembled in the Bunsengesellschaft had other interests. Furthermore we should mention that the Zeitschrift für Elektrochemie was certainly an odd journal for these papers: it was published in German and was known little outside Germany and Austria, particularly by analytical and petroleum chemists who almost immediately picked up the papers of James and Martin published just one year later. The lack of knowledge of the activities in Innsbruck was best illustrated by the fact that even the first edition of the basic textbook on GC by A.I.M. Keulemans published in 1957 (22) completely ignored her work (and in fact, gas adsorption chromatography). This deficiency finally was corrected two years later in the second edition, having now a chapter on gas–solid (adsorption) chromatography; as stated in the preface, the new edition "has now attempted to do fuller justice to the work of Professor Erika Cremer of Innsbruck, Austria, and her school" (23). Meanwhile, she also was asked to translate this book into German, and she added a supplemental chapter to this edition, summarizing their work in Innsbruck. Keulemans emphasized in the preface of the German edition, that she was "particularly predestinated" to add this supplement, since her institute "has been working in gas chromatography since 1946, and she was also the first who accomplished the quantitative separation and analysis of gases" using chromatography (24).
Finally, starting toward the second part of the 1950s Cremer and her school became a recognized center of chromatography research, particularly in Austria, Germany, and the Eastern European countries. She had a number of graduate students with major contributions to chromatography; the best known is probably J.F.K. Huber (Ph.D. 1960), later professor in Amsterdam and Vienna. Cremer officially retired in 1970, but continued to be active in science, maintaining contact with her former Institute and the chromatographic community (25). Her 90th birthday was celebrated in Innsbruck by a two-day international symposium (on June 19–20, 1990); she attended all the lectures and actively participated in the discussions (26). Soon after her birthday, a 42-min movie was made in which she reminisced about her long life and activities. On her 95th birthday, she was surprised by a major present: the Deutsches Museum (the world's largest technical collection) opened a new branch in Bonn, Germany, and there they recreated the GC system used in 1945–1947 by her with Prior (27). By then she finally started to slow down, and she died peacefully on September 21, 1996, four months after her 96th birthday (28).
I shall finish this discussion with a few words about Fritz Prior. After receiving his Ph.D. degree he had been teaching at a technical college, in Innsbruck, and then between 1952 and 1966 he was lecturing on pharmaceutical chemistry at the University of Innsbruck. In 1966, he switched to politics, becoming lieutenant governor of the Province of Tyrol, in charge of education and culture. He died in 1996 (25).
A Tribute
Starting in 1960, I visited Professor Cremer a number of times in Innsbruck until the last year of her life. We had many discussions on her life and works, and the past, present and future of chromatography. She was a most wonderful lady, and a superb scientist. Her memory is preserved in the annals of chromatography development.
Leslie S. Ettre
From 1988 to 2004, "Milestones in Chromatography" editor Leslie S. Ettre was associated with the Chemical Engineering Department of Yale University (New Haven, Connecticut), first as an adjunct professor and then as a research fellow. Previously, he had been with the Perkin-Elmer Corporation for 30 years. He is currently a member of LCGC's editorial advisory board. Direct correspondence about this column to "Milestones in Chromatography," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com
References
(1) A.T. James and A.J.P. Martin, Biochem. J. 48 (1), vii (1951).
(2) A.T. James and A.J.P. Martin, Biochem.J. 50, 679–690 (1952).
(3) G. Hesse, H. Eilbracht, and F. Reicheneder, Liebig's Ann. Chem. 546, 233–252 (1941).
(4) G. Hesse and B. Tschachotin, Naturwiss. 30, 387–392 (1942).
(5) N.C. Turner, Nat. Petrol. News 35, R234–R237 (May 5, 1943).
(6) N.C. Turner, Petrol. Refiner 22(5), 140–144 (1943).
(7) S. Claesson, Ark. Kem. Mineral. Geol. 23A (1), 1–133 (1946).
(8) O. Bobleter, Chromatographia 30, 471–476 (1990).
(9) K. Peters and K. Weil, J. Physik. Chem. A148, 1–26 (1930).
(10) K. Peters and W. Lohmar, Angew. Chem. 50, 1–42 (1937).
(11) A. Kunte, "Adsorption and Desorption of Acetylene and Ethylene on Active Carbon," thesis for the Master's degree, University of Innsbruck, 1944.
(12) G. Hesse, Adsorptionsmethoden in chemischen Laboratorium, mit besonderen Berücksichtigung der chromatographischen Adsorptionsanalyse (Tswett Analyse) (Adsorption Methods in the Chemical Laboratory, with Particular Consideration of the Chromatographic Adsorption Analysis (Tswett Analysis) (W. de Gruyter & Co., Berlin, 1943).
(13) R. Knöpfler, "Investigations on Chromatography," Ph.D. thesis, University of Innsbruck, January 1946.
(14) E. Cremer, Chromatographia 9, 363–366 (1976).
(15) A.J.P. Martin and R.L.M. Synge, Biochem. J. 35, 1358–1368 (1941).
(16) F. Prior, "Determination of the Heats of Adsorption of Gases and Vapors Using the Chromatographic Method in the Gas Phase," Ph.D. thesis, University of Innsbruck, May 1947.
(17) R. Müller, "Application of the Chromatographic Method for the Separation and Determination of Very Small Quantities of Gases," Ph.D. thesis, University of Innsbruck, May 1950.
(18) Oesterr. Chem. Ztg. 50, 161 (1949).
(19) E. Cremer, Z. Elektrochem. 55, 65 (1951).
(20) E. Cremer and F. Prior, Z. Elektrochem. 55, 66–69 (1951).
(21)E. Cremer and R. Müller, Mikrochem./Mikrochim. Acta 36/37, 553–560 (1951).
(22) E. Cremer and R. Müller, Z. Elektrochem. 55, 217–220 (1951).
(23) A.I.M. Keulemans, Gas Chromatography (Chapman & Hall, London, and Reinhold, New York; first edition: 1957; second edition: 1959).
(24) A.I.M. Keulemans, Gaschromatographie (Translated into German and supplemented by E. Cremer, Verlag Chemie, Weinheim, 1959).
(25) G. Oberkofler: Erika Cremer — Ein Leben für die Chemie (Erika Cremer — A Life for Chemistry) (Studien Verlag, Innsbruck-Vienna, 1998).
(26) L.S. Ettre, LCGC 8, 879–880 (1990).
(27) O. Bobleter, Chromatographia 43, 444–446 (1996).
(28) O. Bobleter, Chromatographia 43, 581–582 (1996).

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.













