Approaches for Extracting and Determining Additives, Contaminants, and Low-Molecular-Weight By-Products in Synthetic Polymers
LCGC North America
Howard G. Barth and Ronald E. Majors Liquid–solid extraction is the most popular method, but sometimes modern approaches such as PLE/ASE and MAE are possible.
This month's installment surveys traditional and modern approaches for extracting and determining additives, contaminants, and low-molecular-weight components that may be present in synthetic polymers or polymeric materials. After sample workup, components are typically analyzed by chromatographic methods. This information is needed for product certification to comply with governmental safety and health regulations.

High-purity polymers are typically required for special applications such as implants or prosthetics, pharmaceuticals, electronic devices, and polymer reference standards. Most polymers of commercial interest, however, contain low-molecular-weight additives that are intentionally added to extend the material's serviceable lifetime or alter its properties and performance. There are also nonpolymeric compounds, oligomers, and low-molecular weight components found in the polymer that originate from the polymerization process itself. Lastly, polymeric material can become contaminated during production, handling, storage, or transportation.
Component levels and their identification are needed for product certification, meeting end-use performance criteria, and for ensuring product stability and safety. (Please note that the term "component" is used throughout this survey as a general label for additives, residual solvents, unreacted monomers, oligomers, chain fragments, low-molecular-weight polymerization by-products or reaction side-products, or contaminants.) Because extractables and contaminants may pose health risks, especially if the polymer is processed into film, packaging, or parts that contact food, pharmaceuticals, viable organisms, and biologicals, accurate and precise analytical data are required.
To comply with governmental safety, health, and transportation regulations, it is necessary for agencies and companies to monitor, control, and certify product quality. This month's "Sample Preparation Perspectives" installment surveys approaches for extracting and concentrating polymer additives and side products that may be present in synthetic polymers or polymeric materials for subsequent chromatographic analysis.
The sample preparation process is an important part of the overall analysis and is often the rate-determining and error-prone part of the analytical cycle. Nonporous solid materials such as polymers represent a special sample preparation challenge because many of the additives are located deep within the matrix itself and must be released in a quantitative manner.
Sources of Components Potentially Present in Polymeric Materials Polymer Additives
Most commercial polymers and polymeric materials contain intentionally added components, many of which are small molecules. Additives are essentially used to extend the lifetime of materials by inhibiting polymer aging and deterioration. Additives are also used to alter the chemical or physical properties of polymeric materials, whereas others act as processing aids. A list of their uses and functions is given in Table I.
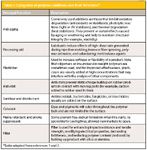
Table I: Categories of polymer additives and their functions*
Additives are usually applied so that they are uniformly distributed throughout the bulk of a polymer, especially at high processing temperatures. Processing aids that help to release formed parts from molds, and finishes applied to fibers to withstand high-speed spinning and winding are examples of components that are applied to the surfaces of materials.
If the solubility parameter, which is related to its polarity of an additive, is greater than the polymer's solubility parameter, additives will tend to "bloom" or migrate toward the polymer's surface with time, decreasing it's effectiveness and causing some surface cloudiness. The degree of blooming depends on the additive's concentration, molecular weight, ambient temperature, the article's size and shape, and the solubility and diffusion coefficient of additive in the polymer.
Components Formed During Polymerization
Polymers may also contain a host of other components left over or formed from the polymerization process, such as residual solvents and surfactants, unreacted monomers or comonomers, linear and cyclic oligomers, and decomposition products of catalysts, initiators, or chain-transfer agents. After the polymerization, polar components may tend to migrate or bloom to the surface.
It is important to realize that with certain types of polymerizations such as random or block copolymerizations, the polymeric material formed may be compositionally heterogeneous, containing polymers that have different chemical compositions or architectures such as branched and cross-linked structures, unreacted blocks or branches, or copolymers enriched in one or the other comonomer. Because of their relatively high molecular weights and unusual compositions, their separation and analyses are indeed challenging (and will not be covered in this column). To complicate matters, most polymers have a molecular weight distribution that can range from oligomers to high molecular weight polymers, depending on the polymerization mechanism.
Contaminants
Polymers in any physical form can become contaminated during or after polymerization, processing, or fabrication. Usually contamination is first realized by the appearance of an unknown peak during chromatographic analysis of a polymer extract. Finding the source of contamination and determining the contaminant's chemical structure, especially at low levels, are indeed daunting and time-consuming tasks. Furthermore, contamination can be quite expensive, especially because of cleanup and disposal. Contamination is usually a surface phenomenon unless it occurs during a polymerization or processing operation such as extrusion.
Typical potential sources and occurrences of polymer contamination are as follows:
- within the polymerization reactor, holding tanks, feed lines, and associated process equipment if any unit was not thoroughly flushed during changeovers and in-between production runs,
- during transportation from plant, fabricator, then to customer's site using trucks, tanks, or railcars not properly cleaned before use, or
- from improper storage in opened or exposed containers.
Because of the huge negative impact that arises from product contamination, it would be highly desirable for chemical and polymer manufacturers to consider setting up additional rapid-response high performance liquid chromatography (HPLC) or gas chromatography (GC) screening facilities at critical points within the plant and nearby loading docks.
Particle Size Reduction of Polymeric Materials
Polymer samples submitted to the analytical laboratory can be of many different physical shapes and sizes. Physical forms can range from pellets, fibers, and irregular pieces, to fabricated finished products — just about any form imaginable. Polymeric materials can be soft (for example, polybutadiene elastomers) to extremely hard (such as polycarbonate pellets) with varying degrees of porosity, surface area, and other physical and chemical attributes. Before extraction and analysis, the polymeric material itself must be changed to a suitable form for further treatment. It is usually desirable to render solid samples into a finely divided state.

Table II: Recommended methods for reducing particle size of polymeric materials
Statistical methods of sampling must be used to obtain meaningful data. Furthermore, samples being compared should have the same average particle size and distribution. For good laboratory practice and obtaining statistically reliable analytical data, it is desirable to comminute and combine samples that have the same production dates and lot numbers. This approach also helps to smooth over discordant data points. When finely divided samples are combined, sample uniformity and homogeneity are ensured, allowing more representative subsampling with greater precision and accuracy. As examined in greater detail in the next section, small-particle-size samples dissolve faster and have larger extraction recoveries because of their greater surface area.
Popular laboratory methods for reducing the size of polymers are listed alphabetically in Table II. Size reduction of a polymer should be carried out at low temperature, preferably well below its glass-transition temperature (Tg), to prevent softening and sticking of the sample. Many of the devices listed in Table II can keep the sample chamber cooled with liquid nitrogen, which is sufficiently cold to maintain the temperature below the Tg of most polymers while pulverizing. The low temperature will also keep samples brittle so that they can easily be pulverized. Because most particle-size reduction techniques will generate a significant quantity of heat, cooling the sample will also prevent possible degradation of additives or the polymer itself, which could lead to spurious results.
The choice of procedure or instrument in Table II is dictated by a number of practical considerations:
- noise level abatement needed for most of these instruments;
- location availability of device, that is, analytical laboratory or plant site;
- sample hardness or degree of softness that can be accommodated;
- particle size range and size distribution that can be generated;
- maximum size and volume of part that can be processed;
- maximum number of samples that can be accommodated;
- maximum throughput that can be accommodated;
- continuous or batch-wise sample processing;
- and sample contamination risk of a given instrument.
Most size-reduction techniques are performed in a dry state, but for certain polymers that tend to agglomerate during grinding, wet or slurry grinding should be considered. However, if a wet or slurry reduction is performed in water or another liquid, precautions must be taken to ensure that particle components are not extracted from the polymer matrix during wet grinding and that no contamination occurs.
Because all comminutive methods involve some sort of abrasion, sample contamination by abrasion particles should not be a concern, unless trace metals are being determined. Good laboratory practices must be strictly followed to prevent contamination of all areas exposed to the polymer sample. This can be done by thoroughly cleaning tubes and magnetic pulverizing devices after each use.
Details on commercially available instrumentation for particle-size reduction are beyond the scope of this article. Nonetheless, readers are referred to references on the subject (3–5) as well as manufacturer's literature. Companies active in this area include Retsch Technology, Spex SamplePrep, Fritsch, and Buehler, among others.
Extracting Components from Polymer Matrices
Removing and determining components of interest in polymers can often be quite demanding. In most instances, components must be separated or removed from the polymer matrix before being analyzed to prevent polymer interference. There are three basic approaches to removing and measuring these components:
- Traditional extraction methods using a thermodynamically good solvent for the components and a thermodynamically poor solvent for the polymer; temperature is used as the main variable
- Modern extraction methods as in the first approach, but using pressure as an added variable
- Complete dissolution of the polymer thereby releasing components; choice of solvent and temperature are variables here.
Tables III and IV provide an overview of traditional and modern techniques, respectively, for component extraction from polymers. The next sections discuss some of these techniques in more detail.
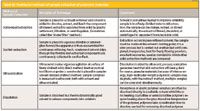
Table III: Traditional methods of sample extraction of polymeric materials
Components found in polymer samples can be either adsorbed on the polymer surface, as in the case of surface contamination, or absorbed within the bulk of the sample, as with additives. Adsorbed components can be either extracted or washed from the surface with an appropriate solvent and analyzed chromatographically. Absorbed components can be extracted from a finely divided polymer with an appropriate solvent. Alternatively, although not an "extraction" technique, the polymer sample can be completely dissolved to release absorbed components from the matrix into solution.

Figure 1: Conceptualized plot of amount of component extracted per total sample weight versus particle size of the sample. Point A represents the maximum amount (or recovery) of a component extracted from a given sample weight at particle size D. Point B is the concentration of the component determined by completely dissolving a given weight of sample.
When the polymer matrix is dissolved on the addition of a good solvent, component molecules become solvated along with the polymer chains. Thus, in principle, the use of a good solvent to dissolve the polymer matrix will also solvate all released components. The analytical challenge is to find the appropriate conditions (that is, solvent and temperature), to dissolve the polymer matrix, and desorb and solvate component molecules. Perhaps the best way of dissolving a polymer is to use a solvent that has a solubility parameter that is numerically close to that of the polymer. For the reader's convenience, Table V lists solubility parameters of commonly used solvents, and Table VI is a compilation of estimated solubility parameters of polymers. The role of temperature is discussed in the following section.
Figure 1 illustrates conceptualized plots of the concentration of extracted additives against particle size of the sample for two cases: adsorbed and absorbed components. If additives are applied only to the surface of the polymer, as in the case of finishes or contaminants, a horizontal line is obtained as shown by Figure 1, line 1a. It is quite obvious that the amount of extracted or recovered component does not depend on particle size; the same results would be obtained whether the extract is from a simple polymer wash or from a comminuted sample.
If, however, components are absorbed throughout the polymer bulk phase, as with polymerized-generated components or the anti-aging additives listed in Table I, then the concentration of extracted components will depend on surface area or particle size, as seen in Figure 1, curve 1b. As the particle size of the sample is reduced, the amount of recovered component will increase asymptotically, reach a maximum at point A, and remain level as the particle size is further reduced to its zero particle size limit at point B. In essence, point B is the concentration of the component as determined by complete dissolution of the polymer in the solvent; that is, at infinitely small particle size (point C). The segment between points A and B represents the finite depth that the extracting solvent penetrates.

Table IV: Modern extraction methods for polymer samples
It should be obvious from Figure 1 that adsorbed components can be determined by a simple wash of the whole or smaller pieces of sample. For absorbed components, complete recovery will be guaranteed only if the sample is dissolved, or if extraction is performed on a finely divided sample measured at point A in Figure 1. This plot has to be determined only once for each polymer-component–solvent combination. After which, single-point extractions can then be determined on all subsequent samples that are reduced to point D particle size.
Traditional Liquid–Solid Extraction
Table III lists traditional or commonly used extraction techniques for removing low-molecular-weight components in polymers, with subsequent chromatographic analysis. Also given in this table is a brief description of polymer dissolution in which thermodynamically good solvents are used to dissolve the polymer matrix.

Table V: Solvent solubility parameters*
Liquid–solid extraction is the method of choice for the removal and subsequent analysis of components that are present on the surface of polymeric materials such as surfactants that are needed for emulsion polymerization, and certain types of additives, like processing aids, fiber and textile finishes, and, of course, contaminants. The major advantages of liquid–solid extraction are that it is a simple procedure to set up, it can be used for rapidly screening and analyzing a large number of samples, and it can be automated. Both Soxhlet and ultrasonic extraction, which are performed with different experimental equipment, are really variations of liquid–solid extraction.
Before extraction, the size of the polymer sample is first reduced by one of the methods given in Table II. The more finely divided the polymer sample, the better the intimate contact between the polymer and the extraction solvent, presumably giving better extraction efficiency.

Table VI: Estimated polymer solubility parameters*
The size-reduced polymer particles are weighed and added to a container with a given volume of solvent. A useful parameter in the choice of a solvent for extraction is its solubility parameter (Table V). It is important to choose a solvent that has a solubility parameter that is close to that of the polymer's solubility (Table VI), but not the same. If it is too similar, the solvent will tend to swell the polymer, making it tacky and difficult to work with. (Of course, if the solubility parameters of polymer and solvent are the same, the polymer will begin to dissolve — perfectly acceptable if that is indeed the goal.)
After the solvent is chosen and added to the polymer, the resulting solution is shaken, stirred, or mixed at a given temperature for a preset period of time. The time it takes to extract the components will depend on the nature of the solvent, extraction temperature, solubility of the components in the solvent, their diffusion coefficients, the particle size, and the degree of agitation. Optimum extraction time must be experimentally determined. Traditional Soxhlet extraction (10) may take a long period of time (hours), but the extraction takes place unattended. Ultrasonic-aided extraction may be shorter because of increased, localized agitation, but its traditional application doesn't use external heating. Conventional heating can be used to speed up the process.

Table VII: Modern solid extraction techniques use higher temperatures and pressures
Modern Liquid–Solid Extraction
Traditional liquid–solid extraction techniques almost exclusively relied on temperature, degree of agitation, and time as the main variables. Thus, quantitative extractions often required a great deal of time. As shown in Table VII, modern liquid–solid extraction devices rely on the added benefit of increased pressure. These instruments now operate at higher temperature and pressure, which increases desorption kinetics and lowers the activation energy of desorption. In addition, higher temperature and pressure facilitates the formation of supercritical fluids, which is a definite advantage of these types of instruments.
During the past decade or so, instrumentation has been developed with new and improved electronics and mechanical design for accurate and precise temperature and pressure control. Modern extraction equipment provides a great deal of automation for improving productivity and providing better quality data, convenience, and ease-of-use. Recent advances of a number of extraction procedures that can be automated are summarized in Table IV. Many of these systems perform extractions in a fraction of the time compared to the older methods. For example, typical pressurized fluid extractions or microwave-assisted extractions may require only 15–20 min of total time. These modern extraction methods have been very popular for the extraction of certain types of solutes in food and environmental samples. It seems only fitting that applications in the polymer field would take hold, which indeed they have.
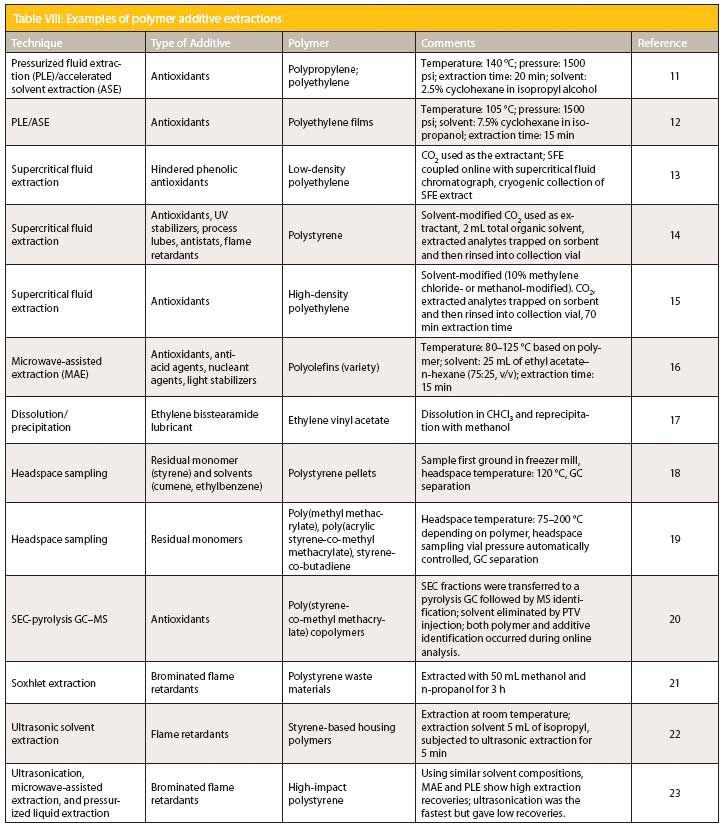
Table VIII: Examples of polymer additive extractions
Because the principles of operation of these modern methods have been previously discussed in this column, we will not delve into the technologies here.
Sample Dissolution
Complete sample dissolution, rather than extraction, is often the method of choice for determining absorbed components that are distributed throughout the polymer matrix, regardless of whether or not "blooming" has occurred. Absorbed components are mainly polymer additives and those produced or left over from the polymerization stage. This approach is valid for samples containing adsorbed components as well.
Polymer dissolution is accomplished by using a solvent that has about the same solubility parameter as the polymer (see Tables V and VI). If the polymer is crystalline or semicrystalline, the solution must be heated to about 50 °C above the melting point of the polymer in order for it to dissolve. During polymer dissolution, components are released from the matrix into solution. Because the resulting solution will be very viscous, and difficult to manage, depending on the molecular weight of the sample, a sufficient volume of solvent must be added to dilute the polymer. If the polymer is cross-linked and insoluble, components will diffuse out from the polymer network into the solvent, which is quite acceptable.
The dissolved polymer fraction, which might interfere with the analysis of sought-after components, can then be removed by adding a nonsolvent or by lowering the temperature of the solution. The precipitated polymer fraction is then removed by filtration or centrifugation.
Alternatively, preparative size-exclusion chromatography (SEC) can be used to separate the polymer fraction from low-molecular-weight components. Preparative SEC has been used quite extensively in chemical, pharmaceutical, and biological industries, including environmental cleanup, and related large-scale separations and purifications. Preparative SEC has been especially useful for the purification of low-molecular-weight components that have been contaminated with high-molecular-weight polymeric material. Because resolution suffers with large-scale analysis, preparative SEC should be used only for fractionation or separation of low-molecular-weight components. The early eluted fractions of polymers should be discarded, and individual later-eluted component peaks collected for analysis or further workup.
Post-Extraction or Post-Dissolution Treatment
After the components are extracted from the polymer matrix, they may need further sample workup before analysis. Although components present in the polymer-free extract can be directly determined chromatographically, it is recommended that the extract be concentrated before injection. This step is accomplished by evaporating the extract to near dryness with a slow stream of nitrogen, or by means of a rotary evaporator. Then, for improved detection limits, the residue should be reconstituted with the smallest possible volume of solvent compatible with the analysis technique (for example, UV, Fourier transform infrared [FT-IR] spectroscopy, or if there is sufficient sample, nuclear magnetic resonance [NMR]). For multicomponent extracts, chromatography with HPLC coupled to mass spectrometry (MS), GC–MS, or SEC are methods of choice. As long as all sample weights and solvent and solution volumes are carefully measured and recorded, concentration levels of identified components can be easily calculated.
For the determination of components in extracts containing dissolved polymers, analytical SEC might prove successful only if the concentrations and detector response factors of components are sufficiently high. Otherwise, these low-molecular-weight components will disappear in the steep tail of earlier eluted polymeric material. In light of these difficulties, as discussed in the last section, the levels of dissolved polymer must be at least partially decreased if not eliminated from extracts to obtain meaningful analytical results of residual low-molecular-weight components.
Volatile Components
Volatile contaminants that are likely to be present in solid polymer samples include residual solvents and unreacted volatile monomers. Volatile samples usually are analyzed by GC. There are many techniques available for volatile contaminants, including gas–solid adsorption, thermal extraction, and gas-phase extraction (such as headspace and purge-and-trap sampling). In these cases, volatiles are sampled above the solid polymeric substances contained in a closed vessel. The most popular methods are headspace sampling and purge-and-trap sampling. In the headspace method, the finely divided polymer is placed in a 20-mL vial at a defined temperature and pressure. The volatiles are allowed to come into equilibrium in the headspace above the polymer and an aliquot of the headspace vapors is injected into the GC for analysis.
In the purge-and-trap approach, sometimes called dynamic headspace sampling, an inert gas continually removes headspace vapors above the solid sample. The flow of gas over the sample will further volatilize analytes that can be trapped by an adsorbent or by cryogenic means. By thermal desorption, the trapped analytes are eluted into the GC column. The purge-and-trap process is particularly useful for analytes that are too low in concentration to be measured by static headspace methods.
Applications of Extraction Procedures
Table VIII provides a number of examples of successful polymer additive extractions. Modern extraction techniques are especially prevalent because they are much faster and more automatable than the traditional extraction techniques. Note the rapid extraction times for the pressurized fluid extraction (PLE) and microwave-assisted extraction (MAE) techniques.
Conclusions
This installment of "Sample Prep Perspectives" provided a discussion on the various approaches to extract and determine nonpolymeric components in polymer samples. These components can vary from additives added to impart favorable properties, unwanted residuals from the synthesis process, and contaminants picked up along the way. Liquid–solid extraction, including both traditional and modern approaches, is still the most popular method. Modern instrumental extraction approaches such as PLE/ASE and MAE are faster, more automatable, convenient, and easy to use. Polymer dissolution methods can also work, but require additional steps to remove dissolved polymer before analysis.
References
(1) D. Walton and P Lorimer, Polymers, Oxford Chemistry Primers (Oxford Science Publishers, Oxford, UK, 2000).
(2) J. Brydson, Plastic Materials, 7th ed. (Butterworth Heinemann, Oxford, UK, 1999).
(3) Sample Preparation Techniques in Analytical Chemistry, S. Mitra, Ed. (Wiley-Interscience, Hoboken, New Jersey, 2003), pp. 458.
(4) http://www.laboratoryequipment.com/articles/2011/06/faster-more-cost-effective-sample-prep.
(5) http://www.chemindustry.com/popular/S/sample_preparation_instruments.html.
(6) Polymer Handbook, 3rd ed., J. Brandrup and E.H. Immergut, Eds. (Wiley, New York, 1989).
(7) Burdick & Jackson Labs., Inc., Solvent Guide, 1980.
(8) D.W. Van Krevelen, Properties of Polymers (Elsevier, Amsterdam, The Netherlands, 1990).
(9) R.B. Seymour and C.E. Carraher, Jr., Polymer Chemistry: An Introductio, 3rd Ed. (Marcel Dekker, New York, New York, 1992).
(10) S. Arment, LCGC North Amer. 17(6S), S38–S42 (1999).
(11) Accelerated Solvent Extraction (ASE) of Additives from Polymer Materials, Dionex Application Note #331, Sunnyvale, California, April, 2011.
(12) A.Garrido-López and M.T. Tena, J. Chromatogr. A 1099, 75–83 (2005).
(13) L.Y. Zhou, M. Ashraf-Khorassani, and L.T. Taylor, J. Chromatogr. A 858, 209–218 (1999).
(14) S.H. Smith, "Extraction of Additives from Polystyrene and Subsequent Analysis," Chemistry MS thesis, Virginia Polytechnic Institute, Blacksburg, Virginia, 1998.
(15) A.M. Pinto and L.T. Taylor, J. Chromatogr. A811, 163–170 (1998).
(16) B. Marcato and M. Vianello, J. Chromatogr. A 869, 285–300 (2000).
(17) K. Gaudin, H. Ho-Sung, J. Bleton, J. Joseph-Charles, P. Dallet, P. Puig, and J.-P. Dubost, J. Chromatogr. A 1167, 27–34 (2007).
(18) W. Shen and C. Wang, "Multiple Headspace Extraction For The Quantitative Determination Of Residual Monomer And Solvents in Polystyrene Pellets Using The Agilent, 7697a Headspace Autosampler," Agilent Technologies Application Note Number 5991-0974en, August, 2012.
(19) R.L. Firor, "Residual Monomers in Polymers by Multiple Headspace Extraction using the Agilent 7697A Headspace Autosampler," Agilent Application Note 5990-0342EN, May, 2012.
(20) E.R. Kaal, G. Alkemab, M. Kurano, M. Geissler, and H.-G. Janssen, J. Chromatogr. A 1143, 182–189 (2007).
(21) M. Riess and R. van Eldik, J. Chromatogr. A 827, 65–71 (1998).
(22) M. Pohlein, A. Segura Llopis, M. Wolf, and R. Ildik, J. Chromatogr. A 1066, 111–117 (2005).
(23) F. Vilaplana, P. Karlsson, A. Ribes-Greus, P. Ivarsson, and S. Karlsson, J. Chromatogr. A 1196–1197, 139–146 (2008).

Howard G. Barth
Howard G. Barth is now retired from the DuPont Company, where he was senior research associate. He is available for consultation in chromatography, analytical chemistry, and technical editing and writing. Please direct correspondence to: howardbarth@gmail.com.
Ronald E. Majors "Sample Prep Perspectives" Editor Ronald E. Majors is a Senior Scientist in the Columns and Supplies Division at Agilent Technologies in Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column via e-mail to lcgcedit@lcgcmag.com.

Ronald E. Majors

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







