Gases Q&A, Part II
LCGC North America
More questions about gases and their delivery to a GC instrument
This month we complete a list of questions about gases that began in an earlier installment.
The range of questions about gases in gas chromatography (GC) is wide and complex. Even barring direct questions about hydrogen as a carrier gas, my attempts to address at least most of the core questions has already taken two installments of "GC Connections." I hope this month's additional topics will suffice, but I also encourage readers to correspond with any other questions they may have on these or any other GC-related subjects.

Clarification on Sealing Tape
More than one reader commented that in the previous column (1), no explicit precaution was given regarding the use of polyfluorocarbon tape or sealant with swaged fittings. The use of any tape or sealant is clearly proscribed for swaged fittings as well as for cylinder compression fittings. A better statement would be that polyfluorocarbon tape specifically sold for high-purity gas distribution is the only type of tube or fitting sealant that can be used for gas chromatography, and the only place that it is appropriate for use is on pipe-thread fittings where it functions as a sealant and prevents gas from flowing around the pipe threads themselves. This applies not only to ¼-or ⅛-in. (6- or 3-mm) tubing and associated fittings but also to larger sizes such as sometimes found in manifolded gas distribution systems (see below for more). Thanks to those readers for helping to clarify this issue.
More Questions That Should Be Asked Frequently
Here are the remaining questions on my list regarding gases and gas delivery for GC. As before, these questions do not address questions about the gases after they reach the GC system. Therein lie even more questions that also should be asked frequently.
What Types of Filters Should Gas Chromatographers Use?
Recommendations for filter types vary for different instrument manufacturers as well as for different consumables suppliers and producers. Filter requirements are driven by the types of injection, column, and detection technology in use. In mixed situations, higher purity requirements override less stringent ones, so always use a filtering scheme that is appropriate for the component that requires the highest purity level. Table I is an expanded version of a table included in the previous installment (1) that gives a filter and gas selection matrix for commonly used inlets, columns, and detectors. Be sure to choose filters that are rated for the desired gas purity level and devices in use.
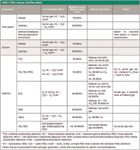
Table I: Filter and gas selection matrix
For example, if using a split–splitless inlet with a wide-bore porous-layer open-tubular (PLOT) column and thermal conductivity detection (TCD), the TCD makeup gas — which has a less strict purity requirement — would be subsumed to the carrier gas due to its higher purity specification. A separate makeup gas supply is not required with TCD; makeup gas can be pulled from the higher-purity carrier-gas supply. An electron-capture detector with a split–splitless inlet, capillary columns, and hydrogen carrier gas would require at least 99.9995% carrier gas and multiple high-purity carrier-gas filters, plus a separate supply of 99.9995% impurity-free nitrogen or argon plus 5% methane ionization gas with its own set of high-purity gas filters.
In general, most chromatographers will simply install the highest level of filtration and use the highest gas purities that apply to all in-use or anticipated combinations of inlet, column, and detector on a particular instrument population.
When Should I Change or Replace My Gas Filters?
Gas filters for GC either include an end-of-life indicator or they are specified to purify a set gas volume. The indicating types are more efficient, in the sense that they don't need to be changed until they indicate the approach of exhaustion. A nonindicating filter that expires after some number of tanks of gas must be changed at that point, whether truly exhausted or not. A nonindicating filter also creates the extra burden of noting for each filter how many tanks of gas have gone through it — assuming of course that it will purify more than one tank. Nonindicating filters usually will include a specification for just how contaminated the incoming gas can be to meet the filter lifetime specification. However, feeding less-contaminated gas — as printed on the gas tank analysis — cannot be assumed to yield a longer filter lifetime. The filter is supposed to remove extra contamination that may be entrained into the gas and not improve on the purity of the gas that is in the tank.
How Do I Leak-Check the Gas Delivery System?
I use three methods to check a gas delivery system for leaks. First, determine if there are any gross leaks by performing a pressure-drop test. Pressurize the gas lines and operate at normal flows at the instrument for a few minutes. Then turn off the flows at the instrument and mark the position of the high-pressure gauge needle by attaching a sticky note to the gauge face with its edge aligned to the needle. Next, turn off the high-pressure tank valve. The high-pressure gauge should remain steady. If it does not then there is a serious leak somewhere. If the gauge appears steady, then wait 5 min. Now, while watching the high-pressure gauge needle in relation to the sticky note, turn the high-pressure valve back on. The gauge needle should remain steady. If it moves upward slightly then there is a low-level leak somewhere.
Even if no apparent leak is indicated by the pressure-drop gauge test, use an electronic leak detector as a second way to verify critical gas lines such as carrier or make-up gas, as well as any hydrogen lines. The pressure-drop test usually will suffice for air lines, as an electronic leak detector does not work for air. If a leak is indicated for an air line, use a few drops of pure liquid water — not soap solution — to try and find it. If you are really concerned, it is possible to pressure the line with helium for the purposes of a leak-check with the electronic meter.
Should I Use Gas Tanks or a Gas Generator?
This question is closely related to the adoption of hydrogen carrier gas. So many have asked about it, however, that I'm including some responses here.
Gas generators provide a high level of convenience over cylinders, but at a higher initial cost; they are an excellent way to replace gas tanks in laboratories that consume them frequently. How often is "frequently"? That depends on the trade-offs between the cost of the individual tanks taken against the cost and maintenance of a gas generator over its useful lifetime. The advantages of gas generators also depend strongly on the real safety and convenience advantages gained from having fewer heavy, high-pressure cylinders to manage. In some situations this advantage alone makes changing to gas generators an easy decision. The following examples are based on full retail prices for gas generators taken from one chromatography supplier's web site in November 2012, and while the numbers are certainly representative, it would be crucial to work with a supplier to arrive at realistic figures for a specific laboratory.
Suppose a 49-L tank of high-purity helium costs $400 in quantity under a supplier contract — if you can find some, of course. A GC carrier-grade hydrogen generator with 500-mL/min capacity might cost $10,000–$15,000. Each full helium cylinder of this size contains around 8 m3 of gas, which would last for about 11 days at a 500-mL/min delivery rate to two or three GC systems with split–splitless inlets. In one year, this laboratory would consume around 32 cylinders of carrier gas for these GC systems, at a cost of $12,800, plus any extra demurrage for keeping spare cylinders on-hand. A more accurate return on investment (ROI) calculation can be obtained by considering the cost of the funds to purchase the generator and the tax advantages its depreciation would produce, but it's clear from the above quick estimate that the hydrogen gas generator saves its cost in helium cylinders within a year or so. Of course, this assumes that the laboratory can replace expensive helium carrier gas with hydrogen within the scope of their operating procedures and methodology. There is a cost of changing to hydrogen because of translating and validating methods, but in most cases this is not too difficult. The good news is that lots of support is available for this one-time activity.
What about a zero-grade air generator — really an air purifier — for flame ionization detectors? This device actually consists of two components: a zero-air purifier and an air compressor to supply it. Together, they might cost around $4000 for a 1-L/min system that could supply two flame ionization detectors. Zero-grade air tanks might cost about $75 a piece, and such a tank would last for about a week at 800 mL/min. So, in a year the cost of the 52 air tanks consumed would be around $3900. Again, ignoring the finer points of an ROI calculation, it is clear that replacing air tanks with an air generator for FID consumption would pay for itself in about one year.
Gas generators do have recurring costs for filter replacements, but this is minor compared to the savings that will result over a 5-year service period for split–splitless carrier gas and FID air generators, not to mention the labor savings and safety gains from not having to haul nearly 100 tanks into and out of the laboratory in a years' time.
When might it not be a good idea to install a gas generator? Consider replacing only the hydrogen tanks used for FID while not switching to hydrogen carrier gas. Around 45 mL/min hydrogen is needed for each flame ionization detector, so the time for a single detector to consume a full hydrogen tank would be about 125 days: that's just three cylinders per year. A hydrogen cylinder for FID use is less than $100, or about $300 per year per detector. By comparison, a smaller capacity 165-mL/min hydrogen generator, which is suitable for three flame ionization detectors, costs about $8000. The three detectors would consume a total of nine cylinders in a year, at around $900 cost, so the ROI period is something on the order of 10 years.
Clearly, the payback on an FID hydrogen-only configuration is not as attractive as the initial gain when moving directly to hydrogen carrier-gas generation from helium cylinders. What about going to hydrogen but without a generator — on cylinders only? Hydrogen cylinders to replace the 32 carrier-gas cylinders in the first example above would cost around $3200, so the net gain in a year compared to using helium tanks would be about $9600. That's close to the cost of a hydrogen generator, so it makes good sense to put the first year and some month's of this savings directly into a hydrogen generator, and save the intermediate transition from helium to hydrogen cylinders for an initial test exercise in method translation, if there are any questions about the switch over.
What About Tanks Kept Outdoors for Gas Distribution Into the Lab?
Many laboratories use an outdoor shed to hold both in-use and spare gas tanks. This is an excellent solution to concerns over housing compressed gas tanks in the laboratory, and this arrangement liberates some laboratory space otherwise occupied by numerous tanks. However, such a setup comes with extra requirements.
First of all, outdoor tanks are subject to extreme temperature swings both daily and as seasons change. Daily temperature fluctuations can drive the internal tank pressure up and down by about 5–7%, while pressure changes could swing as much as two to three times more annually in areas that experience large seasonal temperature changes.
The combination of widely fluctuating tank pressure and temperature affects pressure regulator output levels. Data from outdoor single-stage regulators with outlet pressure monitors that experienced ambient temperatures from -15 °C to +40 °C over the course of six months showed an inverse outlet pressure variation from 86 psig (593 kPa) to 78 psig (538 kPa) in one case, about ±5%. A similar effect was noted for all the observed regulators (2).
A tank heating blanket is another solution for temperature fluctuations at the tank, but usually this is used only on gas-mixture tanks in which a component might liquefy or stratify at cold temperatures. Dual-stage regulators help maintain a more constant output pressure, too, but tank heaters or dual-stage regulators are not necessary for outdoor GC gas tanks, as explained below.
Tank regulators and associated valves and fittings outdoors can be subject to high levels of dust, moisture, and chemical pollution. Brass regulators and conventional pressure gauges — even with nickel flashing on the outer surfaces — can undergo significant degradation in aggressive environments such as near sea water, in a desert location, or in proximity to a chemical plant. Unsealed regulators and gauges are subject to dust and insect intrusion. Any of these can cause the regulator mechanisms to exhibit bias, stick, or even fail.
Beyond regular inspection and maintenance operations to keep regulators in good operating condition, I have encountered a few solutions to the above environmental problems. Installation of outdoor-rated stainless steel regulators and sealed stainless steel gauges is a simple solution, but such devices can be difficult to acquire. A sealed regulator enclosure, with a short flexible high-pressure hose that connects to the gas tank, effectively isolates the regulator from long-term exposure to environmental pollutants. Enclosures specifically designed for this service will have an external safety vent in addition to the inlet and outlet connections. I have also seen sites where the tanks and regulators were located in an enclosed heated gas shed. It is also possible to route high-pressure hoses and piping through outdoor walls to a protected location, but this arrangement requires special materials and skills to properly contain the associated high pressures coming directly from the gas tanks.
The pressure fluctuations in the output of a single-stage outdoors pressure regulator are large enough to cause some supply pressure problems at the gas chromatograph: Why not use a dual-stage regulator? A better solution consists of two single-stage regulators, with one at the tank (outdoors) followed by in-line regulators between the incoming gas line and the backs of the instruments. Set the tank regulator to 150–200 psig (1–1.4 MPa) and set the in-line regulators to deliver 80–100 psig (500–700 kPa) or as required by the instruments.
One feature that is invaluable in outdoor installations is a lock-nut knob-free arrangement for the outlet pressure settings on all regulators. This prevents the inevitable knob-twiddling from no doubt well-intentioned persons. I personally witnessed one such situation where the twiddler said, "Hey, more pressure is better, right?" Another time, someone turned the pressure knob all the way clockwise in a mistaken attempt to turn it off; the result was a broken regulator.
New, cleaned ¼-in. (6-mm ) o.d. copper tubing is a good choice for plumbing between the tank area and the laboratory, if the tubing will not be flexed or disturbed much during its service lifetime. (The metric diameters given here are the commonly available sizes, not the exact equivalent of the fractional sizes.) Larger diameter ⅜-inch (10-mm) tubing is appropriate for runs longer than 50 ft (15 m). Stainless steel tubing is better, but it is also more expensive and difficult to cut and bend. For either tubing material, it's a good idea to connect 6-ft (2-m) lengths of convoluted stainless steel–core hose with a stainless braided outer covering between the tank regulators and the start of the tubing that runs to the laboratory. The flexible links will prevent repeated stress and eventual failure of rigid tubing because of normal tank changes when connected directly to the pressure regulators.
Remember to include a valve and purge-tee arrangement close to the regulator. The purge tee will speed up tank changes by accommodating purging of only the pressure regulator and short tubing in front of the tee. Everyone purges the regulator upon tank changes, don't they? And yes, this is another knob for the twiddlers. Install a lock-out style valve with a padlock if you are seriously concerned.
For high-flow gases such as carrier gas with split injection or air for flame-type detectors, where the total gas flow delivery is greater than a few liters per minute, consider using a tank regulator with a higher flow rating and perhaps even two tanks with an automatic or manual switch over manifold.
Include a pressure gauge, shut-off valve, and another purge-tee arrangement where the incoming gas supply tubing enters the laboratory from the outdoor tanks; this will facilitate purging the long tubing runs from the tanks when necessary. Connect the incoming tubing to a series of tee fittings to distribute the gases to in-line regulators in parallel. Install suitable pressure gauges at the outlets of the in-line regulators if the connected gas chromatographs cannot display incoming gas-line pressures. Additional shut-off or isolation valves at each in-line regulator or, even better, at each instrument will make it easier to install or remove instruments when necessary.
A single large in-line filter is unnecessary if the gas delivery system is well designed, clean, and leak-tight. In fact, a low-grade high-capacity filter might introduce more contamination than it removes at first. However, be sure to include appropriate filters after each in-line regulator, or position individual filters or filter banks close to the back of each instrument.
Conclusions
These two installments on gas Q & A have covered a range of issues about carrier and detector gases, gas distribution, gas generators, filter selection, regulators, tubing, fittings, and connections. Chromatographers should pay careful attention to these areas when setting up or expanding a laboratory, when installing instruments, and of course during routine use. Readers are encouraged to send their comments and additional questions about these or any other GC-related topics to lcgcedit@lcgcmag.com.
References
(1) J.V. Hinshaw, LCGC North Amer. 30(11) 978–981 (2012).
(2) J.V. Hinshaw, personal observations.
John V. Hinshaw "GC Connections" editor John V. Hinshaw is a Senior Scientist at BPL Global, Ltd., in Hillsboro, Oregon, and a member of LCGC's editorial advisory board. Direct correspondence about this column to the author via e-mail: lcgcedit@lcgcmag.com.

John V. Hinshaw

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












