Application of a Risk‑Based Approach to the Migration of Validated Test Methods
The adoption of a risk-based approach to the migration of validated methods in a regulated laboratory is a proactive way of ensuring the actions and control strategy needed for a successful migration to new analytical platforms are in place. Completing this level of due diligence ensures confidence in the performance and equivalency of the new instrumentation by predicting method performance and identifying control strategies, if necessary. For contract research organizations (CROs), this approach to method migration also helps demonstrate to clients that their validated methods will run as intended on new technology platforms, without any need for revalidation.
To mitigate the risks posed by an ageing installed base, laboratories often look to new technology that offers improved performance, reduced downtime, and lower service costs, while faithfully replicating existing validated assays. Consideration of current and forthcoming regulatory guidelines and compliance requirements as well as vendor support are key considerations when evaluating both hardware and software.
Pharmaceutical and biopharmaceutical businesses are now adopting quality by design (QbD) principles to help manage analytical methods throughout their lifetime of use, and regular performance maintenance and the timely upgrade of ageing assets are important aspects of this modern approach. Many contract research organizations (CROs) have an ongoing replacement strategy and budget accordingly for this. However, some make the decision to update ageing analytical instrumentation at short notice because of declining performance and increasing maintenance costs. Poorly functioning or unreliable older instrumentation can increase downtime, leading to compromised timelines, missed targets, and significant costs. Furthermore, such delays and frustrations can also potentially damage a company’s reputation for high-quality customer service. As a result, investments in new technology are usually considered worthwhile before failures start to occur.
Where necessary or beneficial, CROs will work closely with clients to demonstrate that their established methods will run as intended on newer technology platforms, without any need for revalidation. Updated platforms can allow both the laboratory and the customer to keep pace with changing regulatory compliance demands, and improved instrument performance and up time will help ensure deadlines are met and costs are kept to a minimum.
We recently conducted an evaluation to assess the potential impact of upgrading an ageing high performance liquid chromatography (HPLC) fleet on business continuity and to determine whether existing test methods could be migrated seamlessly, and equivalency demonstrated.
Approach
Risk assessment is a key element of QbD. A risk-based approach to the migration of validated methods can help measure the potential impact of differences in instrumentation and software on the performance of the method. This helps companies to make an informed decision when investing in new analytical technology based on the potential cost and time savings provided by technological advances and new software functionality.
The complexity of the risk assessment depends upon the type and extent of differences between instruments and the level of necessary adjustments or controls required to mitigate any potential risk. The process starts prior to the purchase of new technology, with a risk assessment of how instrument differences could impact validated procedures, followed by the creation of testing strategies to determine the severity of the potential impact. Even if the eventual results support the equivalence of the two instruments without the need for the implementation of control strategies, completing this level of due diligence upfront ensures confidence in the performance and equivalency of the new instrumentation.
When properly designed and applied, risk‑based analysis for the migration of legacy methods in a regulatory environment can help users:
- Understand considerations to make when purchasing or upgrading assets;
- Focus on the relevant performance or technical aspects of the instrument specifications;
- Predict the impact of a new instrument on the analytical procedure performance;
- Proactively prevent issues due to instrument differences by making allowable instrument adjustments to align the differences;
- Demonstrate instrument suitability.
Experimental
The phases of the project included:
- Installation of the new HPLC system, plus running instrument suitability and other necessary tests to ensure the system was in operable condition.
- Comparison of the specifications of the current system vs. the new HPLC system.
- Development of risk assessments and control strategies for a specified method (see Figure 1 for the variables included in the risk assessment).
- Demonstration of instrument performance comparability.
- Confirmation that the new instrument’s performance was equivalent and did not contribute to method migration failures, with added controls as determined in the risk assessment.
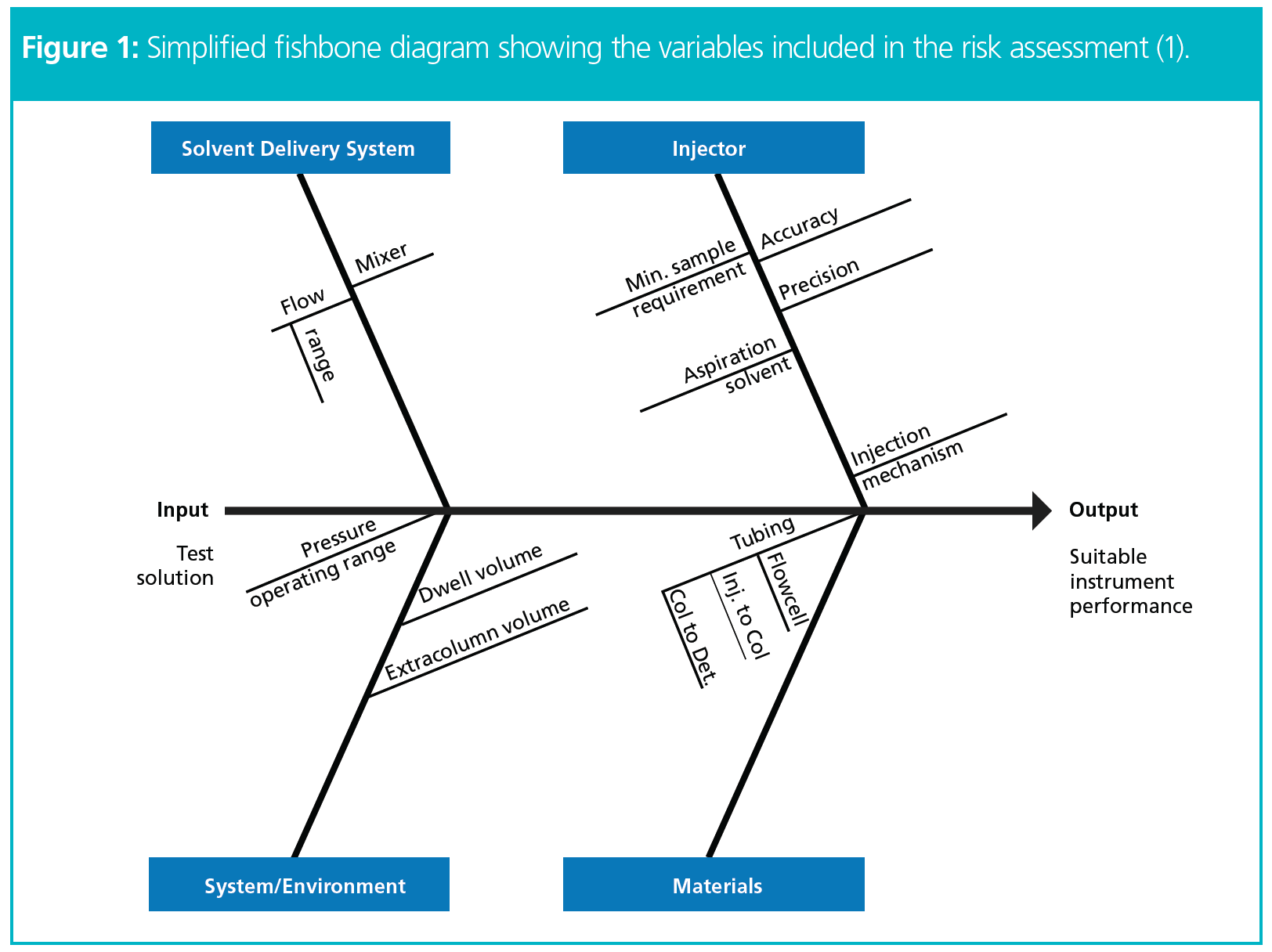
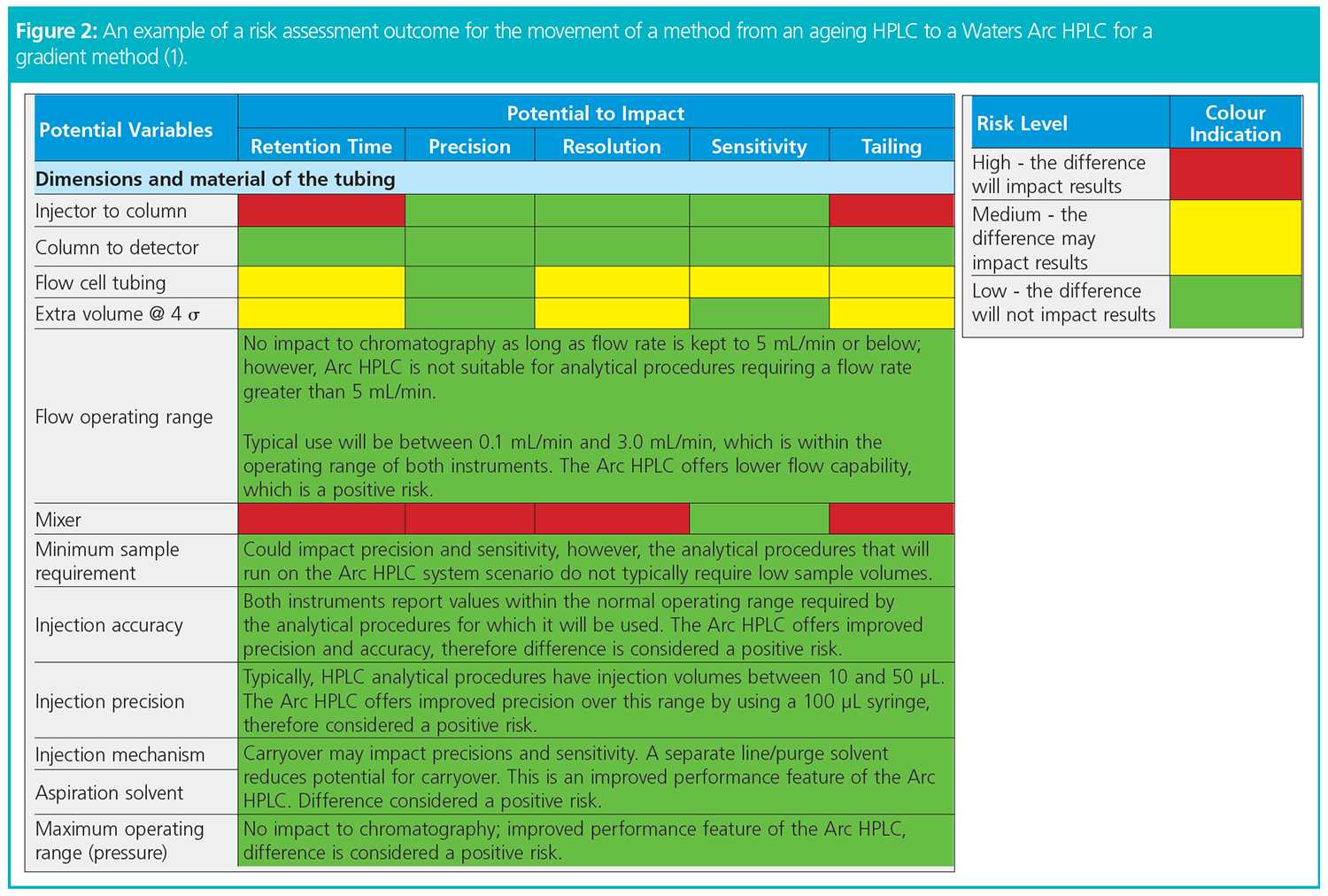
The first step of the project was to compare the specifications of the existing and the new/upgraded instrument to uncover differences that could potentially contribute to inconsistent performance of the analytical procedure once migrated. Next, a thorough risk assessment was conducted to specify each potential variable and to estimate the level of risk (Figure 2). Based on the potential size of the impact and risk level, and using the knowledge gathered, control strategies could be established.
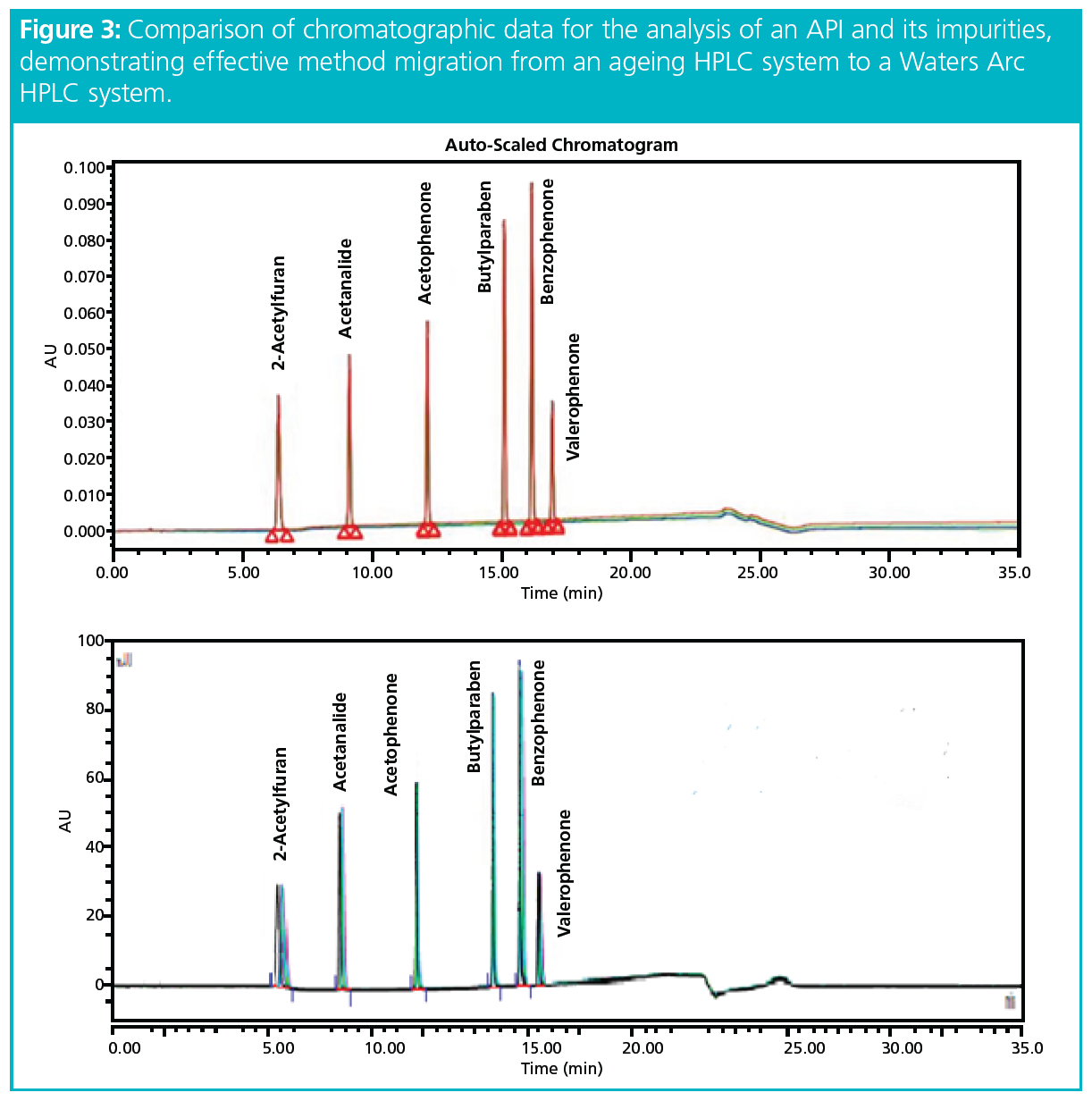
To demonstrate instrument suitability, the testing process duplicated the conditions using the same software (Empower, Waters) for both instruments: the legacy HPLC system from another vendor and the Arc HPLC system (Waters). Evaluating the instruments under identical conditions reduced the risk of variables influencing the testing results. For the comparative testing, the existing equipment and the new HPLC system were run with the same column, the same injections, the same mobile phase, and the same prep for both instruments. The testing was performed side-by-side and back-to-back on the same day to prevent issues with mobile phase differences, sample stability, or column performance. The instrument comparison targeted a 1% difference in the relative standard deviation (RSD) and 3% difference in the absolute retention times.
The control/adjustment strategy was as follows:
- Ensure the analytical methods intended to be moved to the new HPLC have flow rates and injection volumes within the operating ranges of both instruments and do not require low injection volumes;
- Accommodate any differences in tubing at the time of instrument installation;
- Accommodate any minor physical adjustments required for mixing inconsistencies at the time of installation.
Results
The experimental results demonstrated that the new HPLC instrumentation could directly replicate existing LC methods without compromising data quality (Figure 3). Significantly, the data also confirmed that no changes to the original method parameters would be required. The results of this risk‑based approach can be used to document the performance and equivalency of the new instrumentation (1). CROs can also use these results to demonstrate to clients and regulatory agencies that validated methods will run as intended on the new technology platforms, without any need for revalidation.
Conclusion
As long as the analytical procedures targeted to be moved to a new instrument comply with the recommended control strategies identified through the risk assessment, the new HPLC instrumentation could directly replicate existing LC methods without compromising data quality, as demonstrated by the investigation (1).
The risk-based approach to instrument replacement described here can be applied across different models and vendors, with the complexity increasing when different chromatography data systems are used or performance grades of instrumentation vary. Even if the LC platforms are very comparable and the replacement is straightforward, without the need for control strategies, this approach provides confidence in the asset replacement, and analysts will benefit from the improved repeatability and consistency of results offered by more modern HPLC technology.
Reference
- P. McGregor et al., Mitigating Risk of Validated Analytical Procedure Failures When Upgrading or Replacing LC Assets: Harnessing the Power of Quality by Design (QbD) Principles (Waters Corp., 2021).
Douglas J. Turk is Business Unit Manager of Eurofins BioPharma Product Testing in Toronto, Canada. Doug has over 30 years of experience in pharmaceutical R&D and analytical testing in both large pharma and contract research organizations. Doug received a Doctor of Philosophy degree in analytical neurochemistry from the University of Oklahoma in Norman, Oklahoma, USA, as well as a B.Sc. degree (honours) in chemistry and an M.Sc. degree in analytical chemistry, both from McGill University in Montreal, Canada.
Uttam Ghosh is Research and Development Manager at Eurofins BioPharma Product Testing Laboratories. Uttam is responsible for all R&D activities at Eurofins, including method development, method transfer, and validation efforts. Uttam has over 12 years of experience in the pharmaceutical industry, including comprehensive knowledge and implementation of current GMP, GLP, FDA, and CFR 21 Part 11 regulations. Uttam obtained his M.Sc. degree in analytical chemistry from Kingston University, UK.
Stephanie N. Harden is the manager of Waters’ global small molecule pharmaceutical market segment, with 20+ years of experience in product and segment marketing. Stephanie has a passion for enabling world-class pharmaceutical development and has been instrumental in helping customers to transform the way their organizations operate. She is a highly cited author with a Ph.D. in chemistry from the University of Bristol, UK.
E-mail: douglasturk@eurofins.com / uttamghosh@eurofins.com / stephanie_harden@waters.com
Website: www.eurofins.com/contact-us/worldwide-interactive-map/canada/eurofins-biopharma-product-testing-toronto/ / www.waters.com

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.
What Goes in a CDS IT Service Level Agreement?
Published: April 7th 2025 | Updated: April 7th 2025Protecting your network chromatography data system (CDS) data is critical and a service level agreement (SLA) with your IT provider is vital. What should be included? Are SLAs for in-house IT and SaaS (software as a service) similar?
Advanced LC–MS Analysis for PFAS Analysis in Eggs
October 11th 2024The European Commission's regulation on maximum levels for certain contaminants in food highlights the need for precise and reliable methods to quantify per- and polyfluoroalkyl substances (PFAS) in various food matrices. This article discusses development and validation of a robust method for analyzing 21 PFAS compounds in chicken eggs using solid-phase extraction (SPE) and liquid chromatography–mass spectrometry (LC–MS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)