Tips & Tricks: Trouble Analyzing PEGs?
The determination of molar mass and molar mass distributions of polyethylene glycols (PEGs) by size-exclusion chromatography (SEC) is sometimes complicated by experimental artefacts. This instalment of Tips & Tricks will discuss experimental details that need to be considered when analyzing PEGs.
Polyethylene glycols (PEGs) and derivatives have widespread use as raw materials in polymeric products and in medical applications. Therefore, a need exists to characterize their molar masses and molar mass distributions. Gel permeation chromatography/size‑exclusion chromatography (GPC/SEC) is the most important chromatographic separation technique for macromolecules and polymers, and is also applied in PEG characterization. Although the structure of PEGs is simple, GPC/SEC analysis can sometimes create problems if certain experimental aspects are not considered well enough. Problems may arise from sample preparation and from improper selection and treatment of the columns.
Low molar mass PEGs can be dissolved in a variety of solvents, such as chloroform, tetrahydrofuran (THF), dimethyl acetamide (DMAc), methanol, or water. Though PEGs are soluble in principle in THF, DMAc, and other solvents, even low molar masses sometimes dissolve very slowly at room temperature, and a dissolution even overnight is sometimes not successful. However, upon mild heating, the PEGs usually dissolve quickly. For higher molar mass PEGs and poly(ethylene oxides) (PEOs), water is the preferred solvent.
Organic GPC/SEC of PEGs in THF as Mobile Phase
THF is among the most widely applied eluents in GPC/SEC, as it allows a large variety of different polymers to be dissolved. When using THF as an eluent, stationary phases based on styrene–divinylbenzene are often chosen. However, with respect to PEGs, chromatographers often observe distorted peak shapes and non-repeatable chromatograms when using THF on such columns.
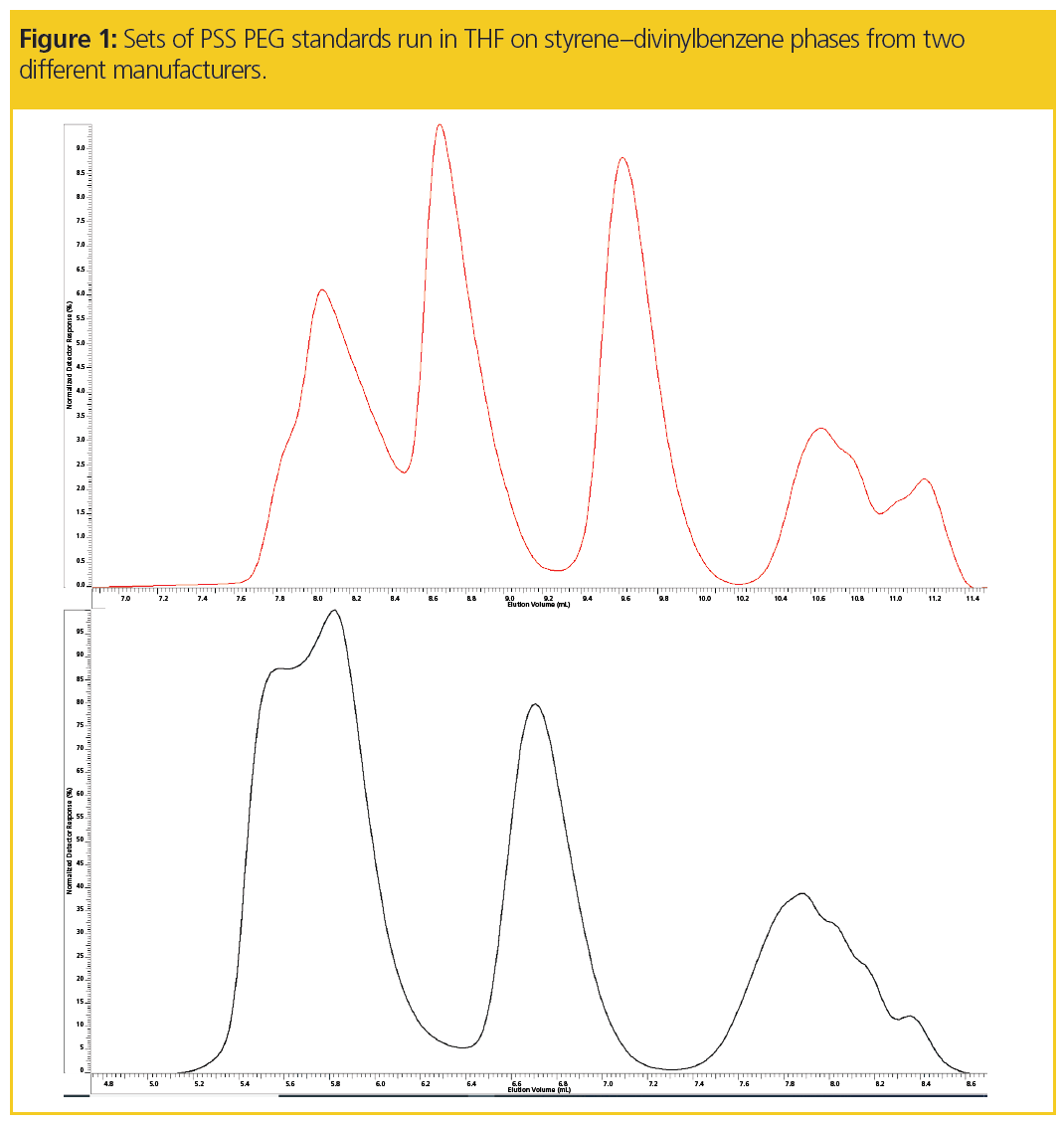
Figure 1 presents chromatograms of PEG standards obtained on styrene–divinylbenzene-based columns from two different manufacturers using pure THF as the eluent. In both cases distorted peaks shapes are observed, particularly for the very high and very low molar masses. However, odd peak shapes are not observed when injecting polystyrene standards under the same conditions. Thus, the columns are generally in good condition and suitable for high performance separations. Therefore, the distorted peak shapes do not result from damaged columns.
The unusual elution behaviour can also be identified by comparing the PEG and polystyrene calibration curves in Figure 2. For both columns, the PEG calibration curve becomes very steep at higher molar masses, which could be misinterpreted as the column’s exclusion limit.
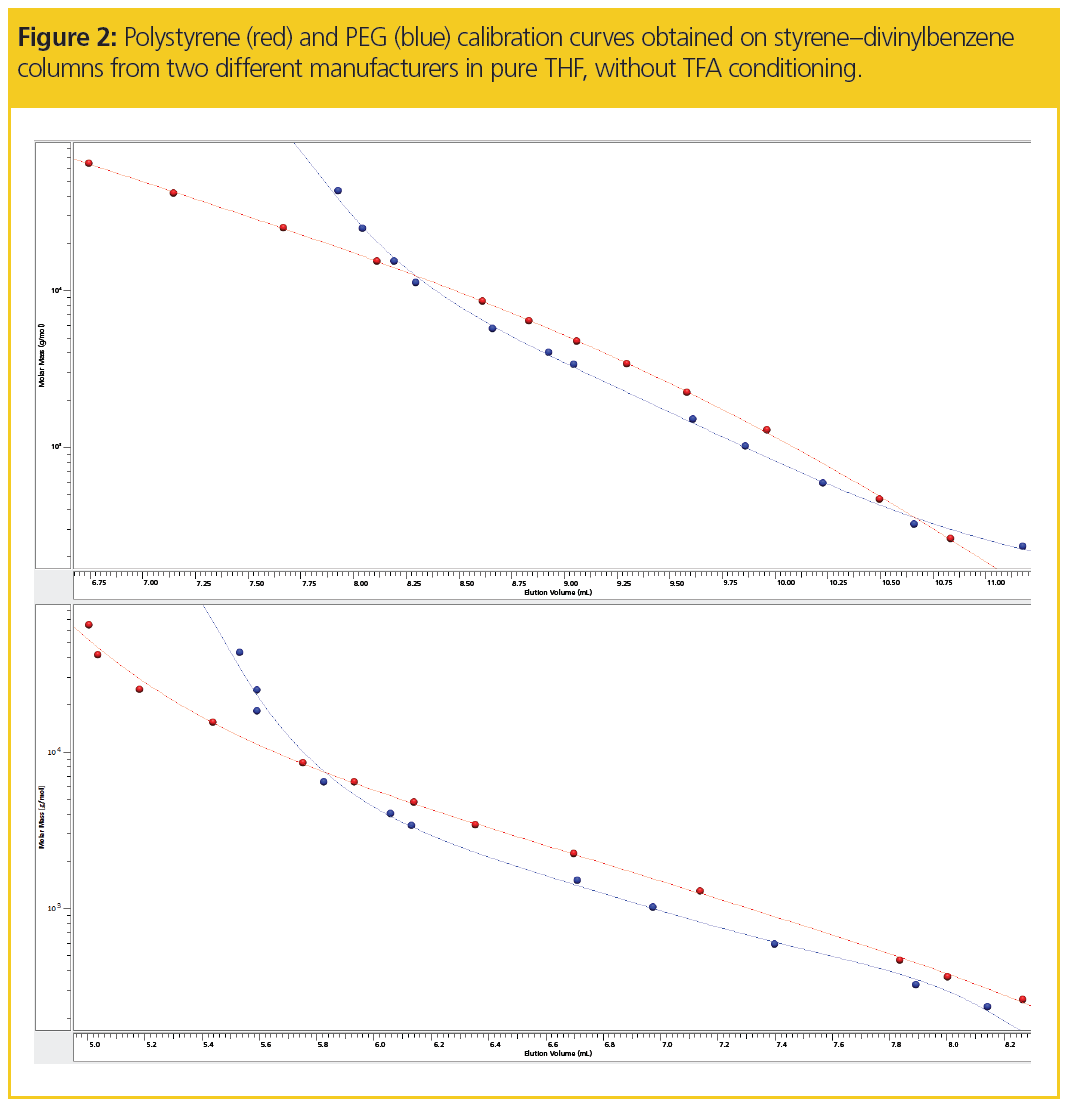
Such interpretation would be incorrect, however, as evidenced by the fact that high molar mass polystyrenes elute significantly before the highest PEG standards. The unusual elution behaviour of the PEGs might be a consequence of undesired interaction with the stationary phase and occurs on columns from different suppliers.
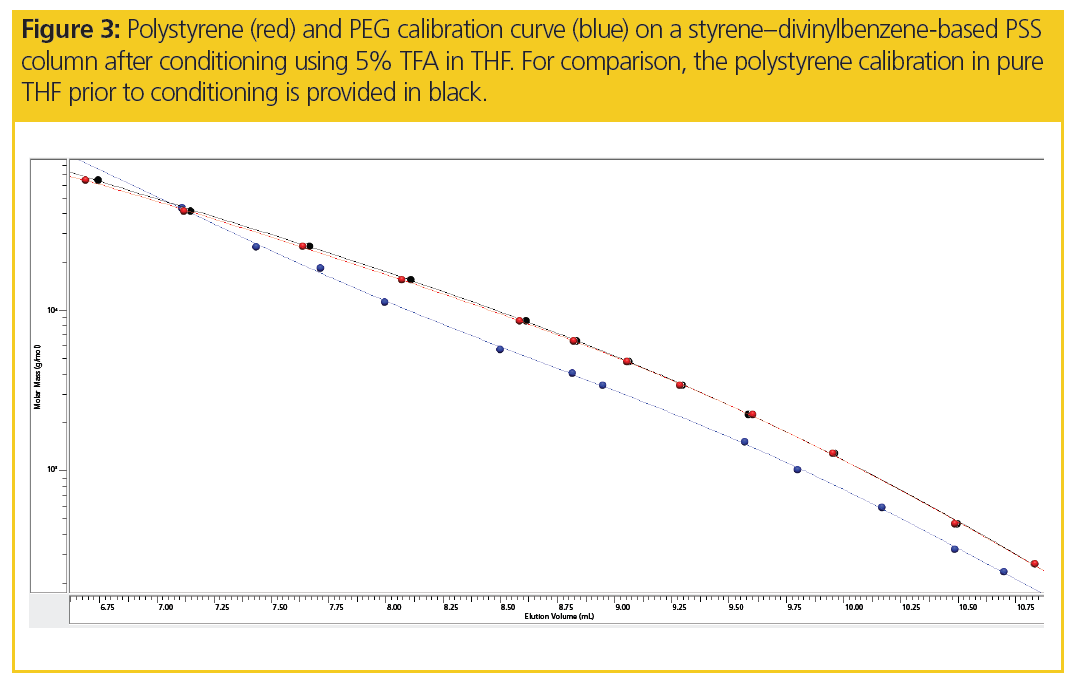
Upon conditioning one column using 5% trifluoro acetic acid (TFA) in THF and reequilibrating the column to pure THF, the PEGs and polystyrene standards are reinjected. While there is only a marginal effect on the polystyrene calibration (see Figure 3), the shape of the PEG calibration differs significantly from those obtained without TFA conditioning.
Not only is the calibration curve significantly altered by conditioning the column, the peak shapes of PEGs are also improved significantly, as seen in Figure 4.
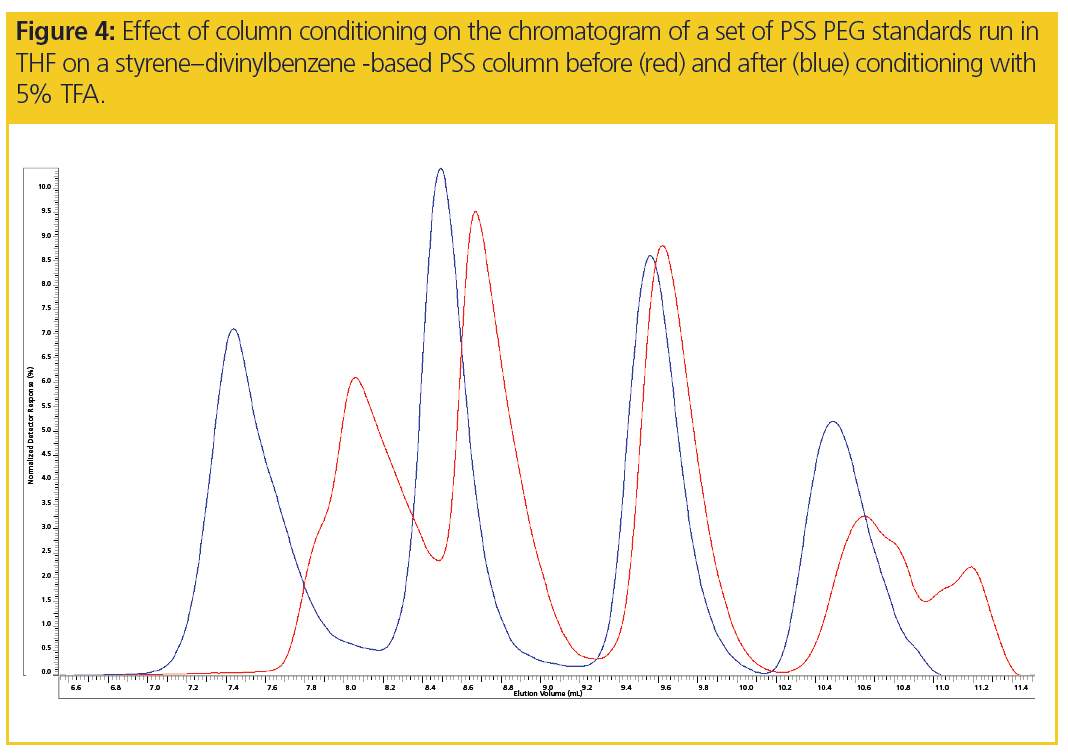
Thus, preconditioning styrene–divinylbenzene-based columns using 5% TFA in THF may allow reliable PEG chromatograms to be obtained.
Aqueous GPC/SEC of PEGs in Water as Mobile Phase
The use of THF as eluent for PEG analysis instead of water, which is far more environmentally friendly and has lower health risks, is most probably due to the earlier development and availability of high-resolution packings for organic GPC/SEC applications compared with aqueous GPC/SEC. Columns for aqueous GPC/SEC with small particle sizes, such as 5 µm or even 3 µm, have only been available in recent years.
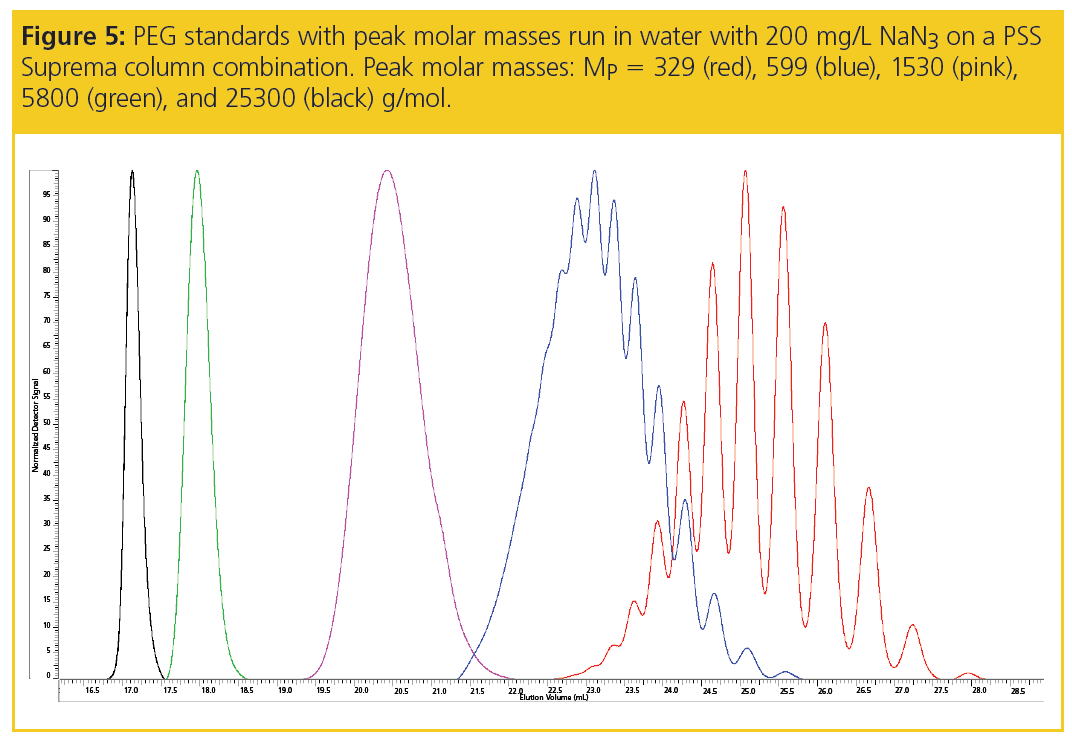
Oligomer resolution of PEGs can now be achieved using modern aqueous GPC/SEC packings, as shown in Figure 5. The PEG oligomers are well separated in water with a small amount of salt. In addition, high molar mass PEGs dissolve in water more easily than in THF. Therefore, if very different molar masses need to be separated or compared, it is advantageous to apply water. Finally, the cost of eluent, the safety, and environmental arguments also advocate for preferring water as the GPC/SEC eluent.
Summary
- Styrene–divinylbenzene-based columns applied for PEG analysis in THF often require preconditioning to obtain suitable and reliable peak shapes.
- Preconditioning using TFA seems to be a suitable means for styrene–divinylbenzene-based columns.
- Modern column materials allow comparable oligomer resolution for PEGs in aqueous GPC/SEC to be obtained, similar to what can be achieved using THF as eluent.
- Often observed slow or incomplete dissolution can be overcome by mild heating.
Wolfgang Radke studied polymer chemistry in Mainz (Germany) and Amherst (Massachusetts, USA) and is head of the PSS application development department. He is also responsible for instrument evaluation and for customized trainings.
Heidi Bergis a trained chemical laboratory technician and has been working in PSS’s contract analysis department on GPC/SEC since 2003.

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Rethinking Chromatography Workflows with AI and Machine Learning
April 1st 2025Interest in applying artificial intelligence (AI) and machine learning (ML) to chromatography is greater than ever. In this article, we discuss data-related barriers to accomplishing this goal and how rethinking chromatography data systems can overcome them.
Influence of Concentration in Conventional GPC/SEC and Advanced Detection GPC/SEC
March 21st 2025Sample concentration is a parameter that can influence the quality of gel permeation chromatography/size-exclusion chromatography (GPC/SEC) separations and the obtained results. Understanding this influence can help to support the development of reliable GPC/SEC methods.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)