The Analysis of Persistent Organic Pollutants Using Pressurized Liquid Extraction and Automated Column Chromatography Sample Cleanup
Special Issues
The persistent nature and toxicity of persistent organic pollutants (POPs) lead to high demands for quick and accurate sample analysis. This article describes the use of automated sample processing techniques such as pressurized liquid extraction and automated column chromatography cleanup that are suitable for environmental sample analysis.
Persistent organic pollutants (POPs) such as polychlorinated dibenzo-p-dioxins (PCDDs), furans (PCDFs), biphenyls (PCBs), polybrominated diphenylethers (PBDEs), and polycyclic aromatic hydrocarbons (PAHs) continue to be analyzed around the world. Their persistent nature and toxicity lead to high demands for quick and accurate sample analysis. This article describes the use of automated sample processing techniques such as pressurized liquid extraction (PLE) and automated column chromatography cleanup that are found to be very suitable for environmental sample analysis. Excellent recoveries, reproducible results, quick turnaround times with same-day results, and closed-loop systems with low background and low power use make the techniques suitable for both academic and commercial production environments. Examples of the analysis of sediment (certified reference material) and soil are given.
The Stockholm Convention on Persistent Organic Pollutants, which has been ratified by most countries in the world, aims at the elimination of persistent organic pollutants (POPs). The convention went into force in 2004. The continued worldwide interest in the compound classes covered by the convention has led to ongoing sample extraction, cleanup, and analysis in many environmental and food laboratories.
Originally, work focused on polychlorinated dibenzo-p-dioxins (PCDDs), furans (PCDFs), and polychlorinated biphenyls (PCBs), but other compound classes such as polycyclic aromatic hydrocarbons (PAHs) and polybrominated diphenylethers (PBDEs) have more recently drawn attention.
PCDDs and PCDFs are unwanted by-products of various processes such as combustion, incineration, metallurgical industry, and pulp and paper bleaching or production. A low natural background from, among other sources, forest fires was suggested in the 1970s (1). PAHs have some sources in common with PCDD and PCDFs, such as internal combustion and incineration. They also occur naturally. PCBs were intentionally produced from the 1920s to the 1970s for use in capacitators and transformers, as flame retardants, hydraulic fluids, sealants, and vacuum pump fluids. Total production has been estimated worldwide at 1.5 million metric tons (2). Similarly, PBDEs have been used and continue to be used as flame retardants around the world.
Although the levels of these compounds have been dropping in more recent years, they continue to be present in the environment and in foods at concentrations significant enough to pose threats to human health. These threats include possible damage to the immune system, the nervous system, and reproductive functions. In addition, they have been identified as endocrine disruptors, carcinogenic, and causing chloracne at higher levels of exposure (3).
These compounds also have low solubility in water and are chemically inert, which is the main reason that they resist environmental degradation. They can be found in many compartments of the environment, such as air, sediment, soil, and water. For environmental samples, the United States Environmental Protection Agency (US EPA) methods such as 1613 (PCDD/Fs), 1614 (PBDEs), and 1668 (PCBs) are generally followed. Because of low concentrations generally found on a per gram of sample basis (often in the nanogram to femtogram range) highly sensitive equipment, such as triple-quadrupole gas chromatography–mass spectrometry (GC–MS) and high-resolution GC–MS, is used for analysis. This analysis requires ultraclean sample processing (extraction, cleanup, concentration) to remove as many interferences as possible.
Experimental
Traditional techniques for sample extraction and cleanup have included Soxhlet extraction and open-column chromatography using various sorbents, such as silica and alumina. Soxhlet extraction can be a lengthy procedure with total extraction times up to 24 to 36 h. Typically, an open system is used so this method of extraction can be prone to background contamination. The use of individual Soxhlet heating elements is also expensive with regards to the consumption of electrical power.
As an alternative, automated methods such as pressurized liquid extraction (PLE) have been developed. A schematic is shown in Figure 1. Any type of solid material can be extracted with this system. A solid or semisolid sample is weighed, mixed with inert drying material (such as Hydromatrix or Ottawa Sand) and placed in a stainless steel extraction cell capped on both ends with PTFE disposable caps. Porous metal frits in the end caps prevent clogging. Various cell sizes of 5–250 mL are used. Samples are usually spiked at this point with relevant internal or isotope dilution standards. The capped cell is placed in the position on the extraction unit and filled from bottom to top with extraction solvent. This solvent is typically 50:50 (v/v) dichloromethane–hexane but other mixtures or solvents can be used. The cell is pressurized at ~1500 psi and heated at 120 °C for about 20 min, cooled for 15 min using a cooling fan, and flushed with extraction solvent (trapped in collection tube) and nitrogen (also in collection tube) to purge out any excess extraction solvent left. The whole procedure takes approximately 45–50 min. All extraction data (pressure and temperature) are computer controlled using real-time software and all events are recorded for traceability. Plots of the parameters are available for automated documentation and data reporting.
Figure 1: Schematic of a pressurized liquid extraction system.
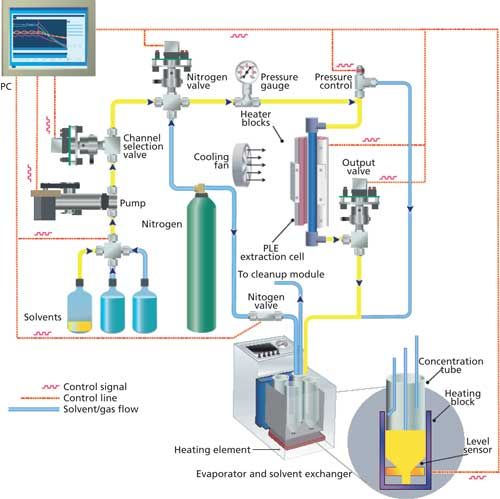
Samples are reduced in volume in a six-position evaporator, which preheats them for 20 min at 60 °C, followed by heating under nitrogen at ~8 psi. The evaporator has built-in sensors that shut off the nitrogen flow when the sample reaches ~0.5 mL of volume. Since the subsequent cleanup requires the extracts to be free of dichloromethane, the samples are solvent exchanged to hexane during the volume reduction.
Further sample processing is accomplished by using automated column chromatography (Figure 2) employing various certified prepackaged columns (silica, carbon, and alumina). The system consists of a control module, valve modules, pump modules, and sample processing modules. A small computer has been included in the system that controls the elution programs. Programming of the various columns, solvent flows, and volumes is done via a plumbing schematic. The system uses flows typically between 5 and 10 mL/min. The columns used are acid-base-neutral silica, carbon, and alumina. This is also the order in which samples are eluted through the system, in contrast to the more typical order silica-alumina-carbon. The silica column has different types of lipid capacity depending on the amount of silica used in the column. The mini version is capable of handling samples with up to 0.2 g of lipid, whereas the high capacity one is able to handle up to 5 g of lipid.
Figure 2: Schematic of a system for automated column chromatography cleanup.
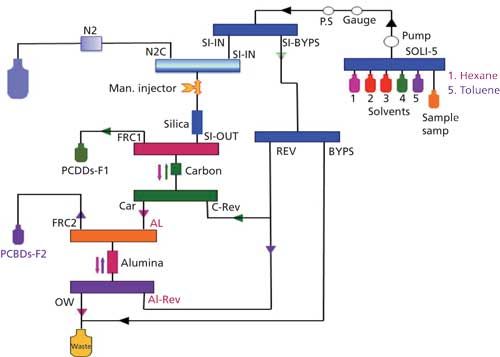
The acid-base-neutral silica will remove components and interferences that can be oxidized or reduced. Alumina serves, among others, to remove chlorinated benzenes. The carbon column retains flat molecules such as PCDDs and PCDFs and co-planar PCBs while letting through mono- and di-ortho PCBs and PBDEs.
The elution program uses hexane and toluene, but no dichloromethane. This is an important consideration as laboratories around the world are trying to phase out this solvent. Columns were first conditioned with hexane. Then the various samples in hexane were loaded automatically onto the system. The silica column was eluted with hexane to transfer the analytes (PCDDs, PCDFs, PCBs) onto the carbon and alumina columns. The volume of hexane used was such that mono- and di-ortho PCBs were passing through the carbon column onto the alumina column. The carbon column was eluted with toluene collecting PCDDs and PCDFs and co-planar PCBs as fraction 1. The alumina column was eluted in the last step with toluene collecting the mono- and di-ortho PCBs as fraction 2. When PBDEs are the desired analytes for collection, these are collected with the PCB fraction in fraction 2. It should be pointed out that the cleanup described uses low volumes of solvent with the totals of hexane and toluene combined between 100–250 mL, depending on which type of silica column is used.
After collection, the sample volume was reduced to 1 mL using the evaporation step discussed above. Samples were then transferred to GC vials and 13C-labeled recovery standard was added. Direct-to-vial connects, which can be screwed onto the collection tubes at one side and onto GC vials at the other side, offer an alternative approach in which sample transfer is not necessary. After the sample is in the GC vial, final volume reduction (typically to 10 µL) is achieved in a 24-position vial evaporator under a gentle stream of nitrogen at ambient temperature.
Analysis is done via splitless injection of 1–2 µL of sample onto the GC column, which is coupled with a Thermo single-quadrupole or triple-quadrupole mass spectrometer (PBDEs) or a Thermo high-resolution magnetic sector instrument (PCDDs, PCDFs, and PCBs). Typically, two ions are monitored for each compound analyzed (multiple ion detection). The temperature programs used usually have starting temperatures at 90–130 °C followed by a relatively steep temperature increase in the beginning and more gradual programming later in the run. PAHs were analyzed on a Thermo Scientific Trace GC system with a DSQ mass spectrometer.
Discussion
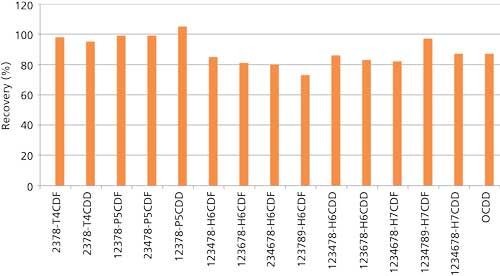
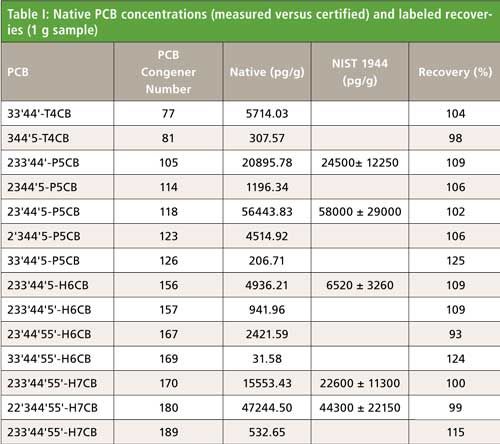
Using the techniques described above, 1 g of New York and New Jersey waterway sediment #1944 certified by the National Institute of Science and Technology (NIST) was analyzed for PCDDs, PCDFs, and PCBs. The results can be found in Figures 3 and 4 and Table I. PCDDs and PCDFs values found in our laboratory were in excellent agreement with the certified concentrations, while giving very good 13C-labeled recoveries of 75–100%. Note that these isotope dilution recoveries showed losses across extraction and cleanup combined. Table I shows very similar data for PCBs.
Figure 3: NIST 1944 certified river sediment analysis (1-g sample).
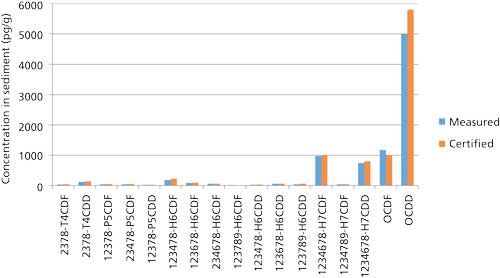
Figure 4: 13C-labeled recoveries for NIST 1944 certified river sediment analysis (1-g sample).
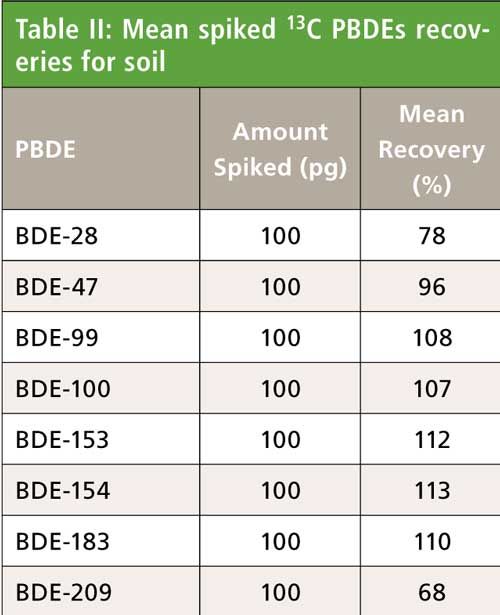
Soil was analyzed as a 10-g sample for PBDEs. The results are shown in Table II. The analysis of eight replicates of soil obtained from the Charles River in Boston, Massachusetts, indicates consistent and reproducible recoveries of 13C-labeled PBDEs. Recoveries fell well within the EPA Method 1614 recovery windows (25–150%), with standard deviations of 2–5% (14% for BDE-209). Native PBDE concentrations detected in samples were at average concentrations of 15.0 ng/kg for BDE 47 and 22.5 ng/kg for BDE-99 (dry weight). Other congeners were nondetect at a reporting limit of 2 ng/kg (20 ng/kg for BDE-209). Evaluation of the multicolumn cleanup procedure on our closed system demonstrated efficient sample processing with low level analysis and excellent reproducibility. The analysis of extracted and cleaned up method blanks verified that the certified prepackaged columns had native PBDEs below instrument detection levels.
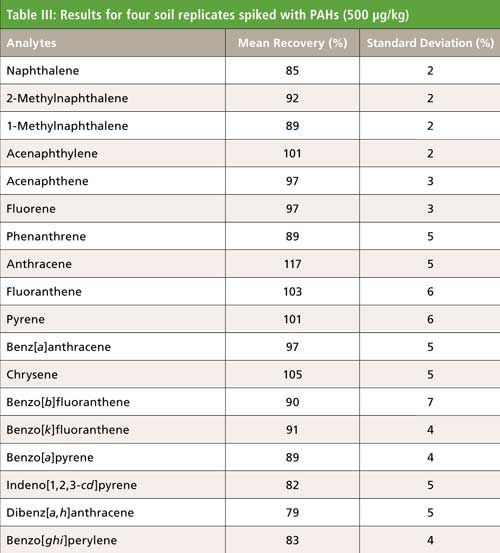
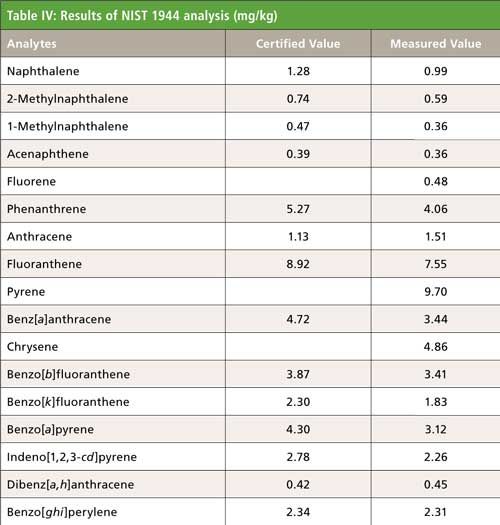
PAHs were analyzed in sediment and soil using automated extraction and clean up across a 6-g neutral silica column (see Tables III and IV). The analysis of the extracts showed excellent extraction efficiencies for all compounds analyzed, with minimal deviation between runs. The calculated concentrations for the NIST 1944 sample were 70–130% for all analytes, thus validating the PLE system extraction for soil and sediment samples. Because of the wide array of other organic contaminants present in the NIST reference sample, the efficiency of the 6-g silica column cleanup was further validated by the clear resolution of each target analyte.
Conclusions
The work presented here shows the feasibility of both automated extraction and sample cleanup (with low solvent use) for environmental samples. Several compound classes of POPs-PCDDs, PCDFs, PCBs, PBDEs, and PAHs-can be analyzed very efficiently and rapidly yielding reproducible concentrations (low standard deviation) and high recoveries. Comparison of measured values with known certified reference materials (CRM) values produced acceptable results within the limits of the CRM. Automated sample processing can be used for method validation (for example, ISO 17025) as fast, same-day results are routinely obtained using these techniques. Extraction (with low electrical power use), cleanup, and analysis by properly trained personnel can be carried out in one day, resulting in low turnaround times for large (and small) sample batches. This fast turnaround is especially important with regards to today’s work environment where customers expect quick results, making this equipment suitable for production laboratories.
References
- R.R. Bumb et al., Science210(4468), 385–390 (1980).
- Persistent Organic Pollutants, S. Harrad, Ed. (John Wiley & Sons, Sussex, UK, 2010).
- M. Kogevinas, Hum. Reprod. Update7(3), 331–339 (2001).
Ruud Addink and Tom Hall are with Toxic Report Laboratories in Watertown, Massachusetts. Direct correspondence to: ruudaddink@toxicreports.com

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.





