Haloacetic Acid Analysis Using Two-Dimensional Matrix-Elimination Ion Chromatography
Special Issues
The disinfectants commonly used to treat public drinking water can react with naturally occurring organic and inorganic matter in the source water to form disinfection byproducts such as haloacetic acids. Here, we describe the use of two-dimensional matrix-elimination ion chromatography (MEIC) for haloacetic acid analysis. This method minimizes the impact of matrix ions.
The disinfectants commonly used to treat public drinking water can react with naturally occurring organic and inorganic matters in the source water to form disinfection by-products such as haloacetic acids. One of the prevailing methods for haloacetic acid analysis is United States Environmental Protection Agency (EPA) Method 552.3, which is a micro liquid–liquid extraction (LLE) method, followed by derivatization, and analysis by gas chromatography (GC) with electron-capture detection (ECD). This analysis is a multistep process and tends to be labor intensive and time consuming. In this article, we showed the application of two-dimensional (2D) matrix-elimination ion chromatography (MEIC) for haloacetic acid analysis. Ion chromatography has been routinely used for ion analysis. However, the large amount of matrix ions that are usually present in drinking water samples can sometimes interfere with the quantitation of trace amount of analytes. The MEIC method described here was able to minimize the impact of matrix ions and provided a direct injection method for nine haloacetic acids (HAA9), which includes HAA5, and four other acids: bromochloroacetic acid, bromodichloroacetic acid, dibromochloroacetic acid, and tribromoacetic acid.
Haloacetic acids are a group of disinfection by-products resulting from the reaction of naturally occurring organic matter and bromide in drinking water with chemical disinfectants that are used in processing drinking water, such as chlorine and chloramine. Long term exposure of these haloacetic acids are known to have adverse effects on human health (1). There are nine haloacetic acid congeners, commonly referred to as HAA9: monochloroacetic acid (MCAA), monobromoacetic acid (MBAA), dichloroacetic acid (DCAA), bromochloroacetic acid (BCAA), dibromoacetic acid (DBAA), trichloroacetic acid (TCAA), bromodichloroacetic acid (BDCAA), chlorodibromoacetic acid (CDBAA), and tribromoacetic acid (TBAA). Out of these nine haloacetic acids, five (MCAA, MBAA, DCAA, DBAA, and TCAA) are currently regulated by the United States Environmental Protection Agency (US EPA). Under the Stage 2 Disinfectants and Disinfection By-products Rule (DBPR) set by the US EPA, the maximum contamination level (MCL) for these five haloacetic acids (HAA5) is set at 60 µg/L (2). A system is in compliance with these MCLs when the locational running annual average for HAA5 at each monitoring location in the distribution system is less than or equal to the above MCL.
The low levels of haloacetic acids are usually present along with a relatively large amount of matrix ions in drinking water and pose an analytical challenge. One of the prevailing EPA methods for haloacetic acid analysis and monitoring is EPA Method 552.3 (3), which is a gas chromatography (GC) method with electron-capture detection (ECD) that provides good detection limits and recovery. However, the haloacetic acids need to be extracted by solvent first and then derivatized by methylation to form methyl esters before GC analysis. The method is prone to operational errors because of the complicated sample preparation involving the multiple-step liquid–liquid extraction (LLE) and derivatization.
Ion chromatography (IC) with suppressed conductivity detection has been extensively used for ion analysis in drinking water and wastewater (4,5). Haloacetic acids are relatively strong acids, which makes them ideal for direct detection with conductivity detection. Several attempts have been made to determine the haloacetic acids with ion chromatography with limited success (6–10). Since the level of haloacetic acids is typically low, a large volume of the sample needs to be preconcentrated onto a concentrator column, before analysis, and for detection. Concentrating large volumes becomes problematic when the concentration of matrix ions in drinking water is large. The matrix ions could be coeluted or overlap with haloacetic acids, shift the retention time, and distort the peak shapes of the haloacetic acids.
A direct injection method that did not require sample preparation was described in EPA Method 557. This method is an ion chromatography, tandem mass spectrometry (MS/MS) method with negative-ion electrospray ionization for the determination of haloacetic acids (11). It takes advantage of a high-capacity ion-exchange column to better accommodate large concentrations of matrix ions and uses post-column matrix diversion to direct the matrix ions away from the mass spectrometer. It also operates the column at 15 °C to minimize the on-column haloacetic acid degradation. However, it requires the use of MS/MS in multiple reaction monitoring (MRM) mode to enhance selectivity and sensitivity. The method was effective in analyzing trace levels of HAA9 in the presence of matrix ions. However, the method was only adapted by laboratories that had MS/MS available.
Recently, a two-dimensional (2D) matrix-elimination ion chromatography (MEIC) method was developed and successfully applied for the analysis of perchlorate and bromate in drinking water and ground water samples (12–15). This approach uses a large-format column in the first dimension to separate the matrix ions from the analytes of interest. After the suppression of the eluent in the first dimension, the cell effluent becomes essentially deionized water and can be enriched onto a concentrator column to focus the ion of interest. The trace ion of interest can be subsequently analyzed in the second dimension by using a small-format column to provide enhanced sensitivity with a concentration-sensitive detector such as conductivity. The above methods used a 4-mm column format for the first dimension and a 2-mm column for the second dimension, thus allowing for a fourfold enhancement in sensitivity. The first- and second-dimension column chemistries were optimized to provide enhanced selectivity for this analysis.
In this work, we extended the application of this MEIC method to haloacetic acid analysis, first HAA5, which is regulated by US EPA, then HAA9. The analytes of interest (HAA5 or HAA9) separated from the first dimension were enriched in the concentrator column and analyzed in the second dimension. A capillary column format was used in the second dimension to achieve comparable sensitivity to EPA Method 557.
Experimental
Instrumentation
A Thermo Scientific Dionex ICS-5000+ ion chromatography (IC) system was used for these analyses. The IC system consisted of a dual pump module (one analytical pump and one capillary pump), a dual eluent generator (EG) module, a dual chromatography compartment (DC) module with two six-port valves and two conductivity detectors, an IC cube cartridge with a six-port valve for the capillary application, and an AS-AP autosampler with a six-port diverter valve. The Thermo Scientific Chromeleon chromatography data system was used for the instrument control, data acquisition, and processing.
The first-dimension analysis used a Dionex IonPac AS24A analytical column (250 mm x 4 mm) and AG24A guard column (50 mm x 4 mm). The column temperature was set at 15 °C, the autosampler temperature was set at 4 °C, and the injection loop was 500 µL. The hydroxide eluent was generated with an EGC-KOH cartridge at a flow rate of 1.0 mL/min. A continuously regenerated anion trap column (CR-ATC) was used to remove any residual contaminants from the eluent. The gradient condition was 7 mM potassium hydroxide from 0 to 12 min, ramped to 14 mM potassium hydroxide from 12 to 32 min, and step changed to 65 mM potassium hydroxide at 32.1 min. An AERS500 4-mm suppressor was operated under the external water mode. A carbonate removal device (4-mm CRD 300) was installed after the suppressor for the removal of carbon dioxide from the carbonate peak in the sample. The addition of the carbonate removal device helped to minimize the amount of carbonate being transferred into the second dimension. The device was regenerated with a recycling 200 mM sodium hydroxide solution delivered by a peristaltic pump. The cell effluent was directed to port 6 on the second six-port valve in the DC module. The second six-port valve, labeled the diverter valve in Figure 1, was used as a selection valve to allow only the ions of interest to be focused onto the concentrator column on the second dimension.
Figure 1: Two-dimensional matrix-elimination ion chromatography setup.
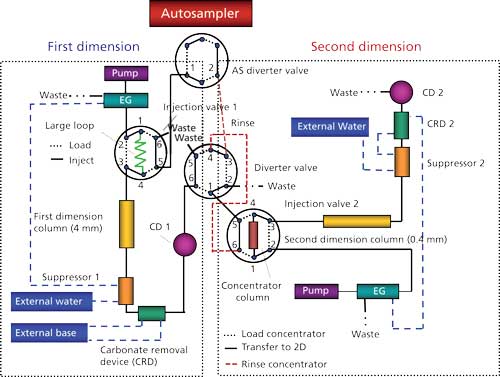
The second-dimension analysis used a Dionex IonPac AS26 capillary analytical column (250 mm x 0.4 mm) and AG26 capillary guard column (50 mm x 0.4 mm). The IC cube cartridge temperature was set at 15 °C and the upper compartment temperature of the DC module was set at 13 °C. A Dionex IonPac MAC-200 concentrator column (80 mm x 0.75 mm) was installed between ports 1 and 4 of the six-port valve in the IC cube cartridge. The hydroxide eluent was generated with a capillary EGC-KOH cartridge at 0.012 mL/min. A capillary CR-ATC was used to remove any residual contaminants in the eluent. The gradient was 5.2 mM potassium hydroxide from 0 to 53 min, step changed to 155 mM potassium hydroxide at 53.1 min, isocratic 155 mM potassium hydroxide from 53.1 to 60 min, and step change to 100 mM potassium hydroxide at 60.1 min for HAA9 analysis. An ACES 300 capillary suppressor was operated under the external water mode. A capillary carbonate removal device (0.4-mm Capillary CRD 200) was installed after the suppressor for the removal of carbon dioxide from the carbonate peak in the sample. The capillary carbonate removal device was regenerated with the suppressor waste.
A detailed schematic for the 2D MEIC setup is shown in Figure 1. Port 1 on the DC diverter valve was connected to port 5 on the injection valve of the IC Cube cartridge using a piece of 0.01-in. i.d. tubing. Port 4 of the DC diverter valve was connected to port 6 on the injection valve of the IC Cube cartridge using a piece of 0.020-in. i.d. tubing. Port 1 on the AS diverter valve was connected to port 5 on the DC injection valve for sample loading, and port 2 on the AS diverter valve was connected to port 3 on the DC diverter valve for delivering the cleaning and reconditioning solutions to the concentrator column. The length of all these tubing was kept to a minimum.
Reagents and Samples
Deionized water (18.2 MΩ-cm, Millipore) was used for eluent and sample preparation. A certified haloacetic acid test mix of 1000 µg/mL each of the nine haloacetic acids in methyl tert-butyl ether was purchased from Restek and used to prepare calibration standard solutions and to perform spike-and-recovery studies. The calibration standard solutions were prepared by serial dilution of the certified mix. For the single-laboratory lowest concentration minimum reporting level (LCMRL) determination, the calibration ranged from 0.05 µg/L to 2.0 µg/L. For the determination of haloacetic acids in water, the calibration ranged from 0.5 µg/L to 20 µg/L. A certified haloacetic acid mix of 2000 µg/mL each of the nine haloacetic acids in methyl tert-butyl ether was purchased from Sigma-Aldrich, and used as a secondary source to validate the calibration curve. All glassware and sample vials were thoroughly cleaned with deionized (DI) water and allowed to drain before usage.
Ammonium chloride was used as a preservative reagent to remove any residual chlorine in the drinking water samples. A stock solution of 100 mg/L ammonium chloride was prepared by diluting 0.1 g of ammonium chloride in DI water to a final volume of 1000 mL. All samples and standards were spiked with 100 mg/L of ammonium chloride.
Laboratory synthetic sample matrix (LSSM), as specified by US EPA method 557, was chosen to represent an upper ionic strength limit for most of the drinking water in the public drinking water systems. It consisted of 250 mg/L chloride and sulfate, 150 mg/L bicarbonate, and 20 mg/L nitrate, and was prepared from the corresponding salts. Ammonium chloride was also added to the LSSM.
The drinking water samples were obtained from various public drinking water sources following the standard EPA protocols. Ammonium chloride was added to achieve a final concentration of 100 mg/L. All samples were stored at 4 °C and analyzed within 14 days.
Cut Window Setting
The cut window is a critical parameter to achieve good recovery of the analyte peaks. This defines the optimal time to capture all the analytes of interest. For this application, three cut windows were chosen. The first cut window included MCAA and MBAA, the second cut window included DCAA, BCAA, and DBAA, and the third cut window included TCAA for HAA5 analysis and TCAA, BDCAA, CDBAA, and TBAA for HAA9 analysis.
To determine the cut window, a solution of 1-mg/L HAA9 sample in reagent water and a solution of 1-mg/L HAA9 sample in LSSM were injected sequentially into the first dimension. The start time for the first cut window was set at 0.5 min before the start of the MCAA elution in the LSSM and the end time for the first cut window was set at 0.2 min after the end of the MBAA elution in the reagent water. The start time for the second cut window was set at 0.5 min before the start of the DCAA elution in the LSSM and the end time for the second cut window was set at 0.2 min after the end of the DBAA elution in the reagent water. The start time for the third cut window was set at 0.5 min before the start of the TCAA elution in the LSSM and the end time for the third cut window was set at 0.2 min after the end of the TCAA elution in reagent water for HAA5 analysis, and after the end of the TBAA elution in reagent water for HAA9 analysis. The cut window was monitored weekly and adjusted as needed.
Sample Overlapping
As the number of analytes increases, the run time also increases. Running both dimensions sequentially would increase the overall analysis time. A sample-overlapping approach was used to shorten the overall run time. The system was separated into two timebases. The second-dimension analysis would only start after the conclusion of all three cut windows. After the analytes had been eluted off the concentrator column (10 min), the concentrator column was switched back to the load position, cleaned, and reconditioned before the start of the first-dimension analysis for the next sample. This approach minimized the transfer of residual contaminants from the first dimension to the second dimension, extending the lifetime of the capillary column.
Concentrator Conditioning
To minimize the carryover from the previous injection and recondition the concentrator column for the next sample, the concentrator column was sequentially washed with 1 mL each of 100 mM sodium hydroxide and 100 mM acetic acid before the start of each analysis. The sodium hydroxide and acetic acid solution were prepared from the corresponding concentrated solutions.
Results and Discussion
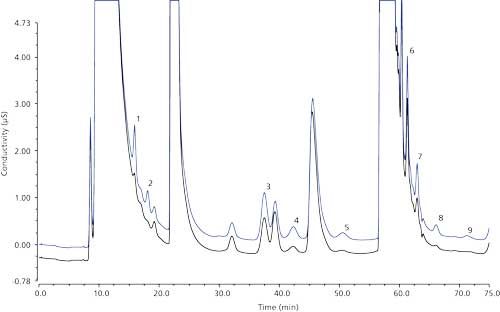
Figure 2 shows the determination of the three cut windows using the 1-mg/L haloacetic acids in reagent water and 1-mg/L haloacetic acids in LSSM. As expected, the presence of large concentrations of matrix ions shifted the retention time and distorted the peak shape of the analyte peaks, as evidenced by broad humps for MCAA and MBAA peaks. The arrows in the graph show the start and end time of the cut windows. The first cut window was ~6 min, the second cut window was ~9 min, and the third cut window was ~3 min for HAA5 analysis, and ~15 min for HAA9 analysis.
Figure 2: Setting the start and end times for the three cut windows in the first dimension using a 1000 µg/L fortification in the reagent water (black trace) and LSSM (blue trace). Peaks: 1 = MCAA, 2 = MBAA, 3 = DCAA, 4 = BCAA, 5 = DBAA, 6 = TCAA, 7 = BDCAA, 8 = CDBAA, 9 = TBAA.
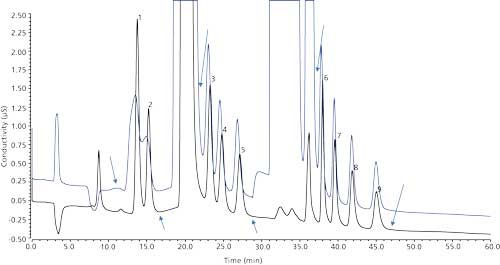
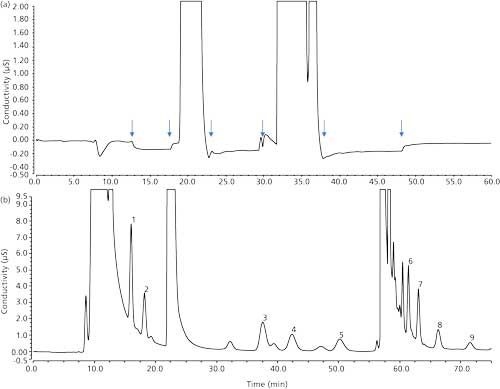
Figure 3 shows the analysis of 20 µg/L of HAA9 in LSSM in the first and second dimensions. The peaks were barely visible in the first dimension, but were clearly identifiable and quantifiable in the second dimension. Almost all of the matrix ions were eliminated.
Figure 3: First and second dimension chromatogram of 20 µg/L HAAs fortifications in the LSSM. Peaks: 1 = MCAA, 2 = MBAA, 3 = DCAA, 4 = BCAA, 5 = DBAA, 6 = TCAA, 7 = BDCAA, 8 = CDBAA, 9 = TBAA.
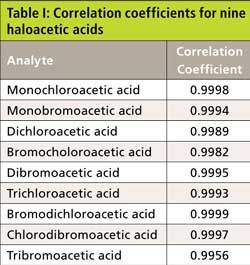
The calibration curve for the nine haloacetic acids was generated using quadratic fit. The correlation coefficient ranged from 0.9956 to 0.9999, as shown in Table I. Three samples of 5.0 µg/L haloacetic acids prepared from the second source were analyzed. The peak recovery ranged from 83.7% to 105.0%, and the percent relative standard deviation (%RSD) ranged from 0.41% to 2.3%, well within the quality data range, confirming the validity of the calibration curves.
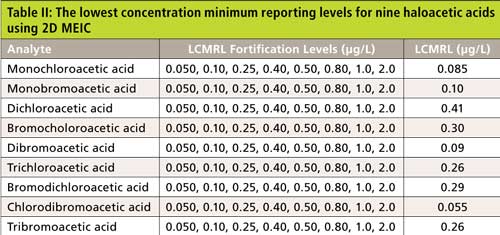
The single-laboratory lowest concentration minimum reporting level (LCMRL), as defined by US EPA (16), was then determined to measure the method detection limit. The analysis batch started with a reagent water blank (containing the preservation solution) and was followed by four replicates of the eight LCMRL standards, as shown in Table II. The detection limits were comparable to those determined by EPA Method 557.
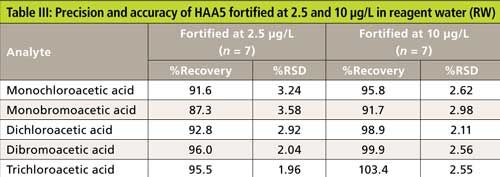
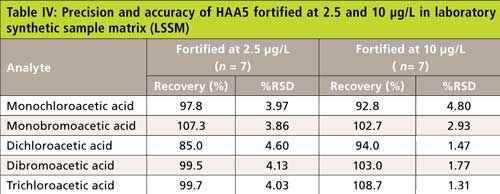
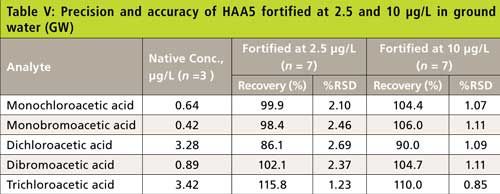
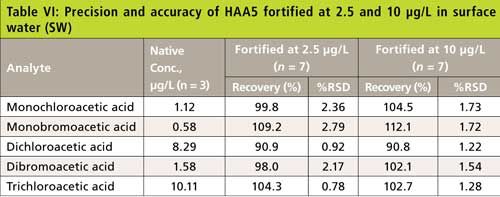
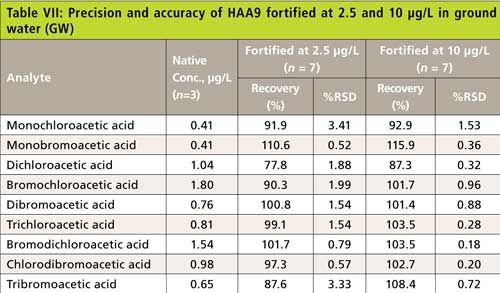
The precision and recovery study was also performed using reagent water, laboratory synthetic sample matrix, ground water, and surface water. The water samples were fortified with 2.5-µg/L and 10-µg/L haloacetic acids. Figure 4 shows typical chromatograms from the second dimension when the sample was fortified with haloacetic acids. The impact of matrix ions in water was minimal. Tables III–VI show the recovery data for HAA5 and Table VII shows the recovery for HAA9. Overall, the recovery was well within ±25% and the %RSD was <5% for all water samples.
Figure 4: Second dimension chromatogram of a municipal surface water disinfected with chlorine and fortified with 2.5 µg/L HAAs. Peaks: 1 = MCAA, 2 = MBAA, 3 = DCAA, 4 = BCAA, 5 = DBAA, 6 = TCAA, 7 = BDCAA, 8 = CDBAA, 9 = TBAA.
Conclusion
In this article, we show the application of two-dimensional matrix elimination ion chromatography for the analysis of five and nine haloacetic acids. This method extended the application of MEIC to multiple-analyte analysis and provided comparable results to that of EPA Method 557, which required tandem mass spectrometry. The present method is also a direct-inject method, avoiding the lengthy LLE and derivatization required for EPA Method 552.3. The use of sample overlapping shortened the total run time. Overall, this 2D MEIC method provides a viable solution for haloacetic acid monitoring for drinking water applications.
Acknowledgments
We gratefully acknowledge the help and encouragement of Steve Wendelken from the US EPA throughout this study.
References
- EPA Integrated Risk Information System, www.epa.gov/iris.
- United States Environmental Protection Agency, National Primary Drinking Water Regulations: Stage 2 Disinfectants and Disinfection Byproducts Rule; final rule, Federal Register, 71, 388–493 (2006).
- M.M. Domino, B.V. Pepich, D.J. Munch, P. S. Fair, and Y. Xie, US EPA Method 552.3, Rev 1.0 (U.S. Environmental Protection Agency, Washington, D.C., 2003).
- J.D. Pfaff, US EPA Method 300.0, Rev 2.1 (U.S. Environmental Protection Agency, Washington, D.C., 1993).
- D.P. Hautman and D.J. Munch, US EPA Method 300.1, Rev 1.0 (U.S. Environmental Protection Agency, Washington, D.C., 1997).
- V. Lopez-Avila, Y. Liu, and C. Charan, J. AOAC Int. 82, 689–704 (1999).
- C. Sarzanini, M.C. Bruzzoniti, and E. Mentasti, J. Chromatogr. A850, 197–211 (1999).
- Y. Liu and S. Mou, Microchem. J.75, 79–86 (2003).
- L. Barron, P. N. Nesterenko, and B. Paul, J. Chromatogr. A1072, 207–215 (2005).
- M.C. Bruzzoniti, R.M. De Carlo, K. Horvath, D. Perrachon, A. Prelle, R. Tofalvi, C. Sarzanini, and P. Hajos, J. Chromatogr. A1187, 188–196 (2008).
- A.D. Zaffiro, M. Zimmerman, B.V. Pepich, R.W. Slingsby, R.F. Jack, C.A. Pohl, and D.J. Munch, US EPA Method 557, Ver. 1.0 (U.S. Environmental Protection Agency, Washington, D.C., 2009).
- R. Lin, B. De Borba, K. Srinivasan, A. Woodruff, and C.A. Pohl, Anal. Chim. Acta567, 135–142 (2006).
- H.P. Wagner, B.V. Pepich, C. Pohl, D. Later, K. Srinivasan, R. Lin, B. De Borba, and D.J. Munch, J Chromatogr A1155(1), 15–21 (2007).
- H.P. Wagner, B.V. Pepich, D. Later, C. Pohl, K. Srinivasan, B. De Borba, R. Lin, and D.J. Munch, US EPA Method 314.2, Rev 1.0 (U.S. Environmental Protection Agency, Washington, D.C., 2008).
- H.P. Wagner, B.V. Pepich, C. Pohl, K. Srinivasan, B. De Borba, R. Lin, and D.J. Munch, US EPA Method 302.0, Rev 1.0 (U.S. Environmental Protection Agency, Washington, D.C., 2009).
- D. Munch and P. Branson, EPA Document #815-R-05-006 (U.S. Environmental Protection Agency, Washington, D.C., 2004).
Rong Lin, Carl Fisher, Kannan Srinivasan and Chris Pohl are with Thermo Fisher Scientific in Sunnyvale, California. Herb Wagner is an independent consultant in Brooksville, Florida. Direct correspondence to: rong.lin@thermofisher.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







